We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1111
From Proteopedia
(Difference between revisions)
| (60 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
{{Sandbox_ESBS_2019}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_ESBS_2019}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | == | + | ==1 PSR == |
| - | <StructureSection load=' | + | <StructureSection load='1psr' size='360' side='right' caption='Caption for this structure' scene=''> |
| - | + | ||
| - | + | ||
== Generalities == | == Generalities == | ||
| - | The structure 1PSR is found in the human psoriasin, also called S100A7. This protein belongs to the family of [http://proteopedia.org/wiki/index.php/Psoriasin S100] proteins. It is a family of 21 proteins of low molecular weights. Those proteins are found in cells as homo and heterodimers. One of their main properties is their ability to bind the calcium. They share some common structures such as two helix-loop-helix structures which are calcium-binding domains. All the S100 proteins have different functions in many various cell types. They have significant roles in calcium-associated signal transduction. They play the roles of calcium sensors proteins that regulate the function or distribution of specific target proteins. | + | |
| + | The structure <scene name='82/829364/1psr/1'>1PSR</scene> is found in the human psoriasin, also called [https://en.wikipedia.org/wiki/S100A7 S100A7]. This protein belongs to the family of [http://proteopedia.org/wiki/index.php/Psoriasin S100] proteins which are involed in the skin celle division and differenciation. They are called like this because of their solubility in a saturated solution with ammonium sulfate at neutral pH <ref> Sedaghat F, Notopoulos A (October 2008). "S100 protein family and its application in clinical practice". ''Hippokratia''. '''4''': 198-204. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2580040/pdf/hippokratia-12-198.pdf </ref>. It is a family of 21 proteins of low molecular weights with a high frequence of homologous sequence (often between 40% and 50%). Those proteins are found in cells as homo and heterodimers. Each monomer have a similar structure, beginning with the N-terminal EF Hand and the four-turn <scene name='82/829364/Helix_1/2'>Helix I</scene> which leads to a <scene name='82/829364/Loop/1'>loop</scene>. Then, there is the three-turn <scene name='82/829364/Helix_ii/1'>Helix II</scene>, the two-turn <scene name='82/829364/Helix_iii/1'>Helix III</scene> and finally the five-turn <scene name='82/829364/Helix_iv/1'>Helix IV</scene>. One of their main properties is their ability to bind the calcium. They share some common structures such as two helix-loop-helix structures which are calcium-binding domains more specifically the loop from <scene name='82/829364/Calcium_binding_site/1'>Asp62 to Asp70</scene>. All the S100 proteins have different functions in many various cell types. They have significant roles in calcium-associated signal transduction. They play the roles of calcium sensors proteins that regulate the function or distribution of specific target proteins <ref>Eckert R, Broome AM, Ruse M, Robinson N, Ryan D, Lee K (July 2004). "S100 Proteins in the Epidermis". ''Journal of Investigative Dermatology''. '''123'''(1): 23-33.https://doi.org/10.1111/j.0022-202X.2004.22719.x</ref>. | ||
== Human Psoriasin == | == Human Psoriasin == | ||
| - | The protein S100A7 is found in the cytoplasm of keratinocytes. The expression of the psoriasin is regulated by different agents. An increase of the cellular amount of calcium also increases the cellular level of S100A7. The UV light increases the expression of the protein. The level of the protein is thought to increase in keratinocytes in response to inflammatory stress. Some studies show that the target protein of the psoriasin could be E-FABP, the epidermal [https://proteopedia.org/wiki/index.php/Fatty_acid-binding_protein fatty acid binding protein]. | + | |
| + | The protein S100A7 is found in the cytoplasm of keratinocytes and on the chromosome 1q21. S100A7 is highly homologous to S100A15 (koebnerisin) but distinct in expression, tissue distribution and function. The protein has a molecular weight of 23 kDa. The expression of the psoriasin is regulated by different agents. An increase of the cellular amount of calcium also increases the cellular level of S100A7. The UV light increases the expression of the protein. The level of the protein is thought to increase in keratinocytes in response to inflammatory stress. Some studies show that the target protein of the psoriasin could be E-FABP, the epidermal [https://proteopedia.org/wiki/index.php/Fatty_acid-binding_protein fatty acid binding protein]. They proved that E-FABP immobilized on nitrocellulose was able to capture S100A7 and so that those two molecules were probably able to form a complex <ref> Hagens G, Masouyé I, Augsburger E, Hotz R, Saurat JH, Siegenthaler G (April 1999) "Calcium-binding protein S100A7 and epidermal-type fatty acid-binding protein are associated in the cytosol of human keratinocytes" ''Biochem J''. '''339'''(2): 419-427: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1220173/</ref>. Fatty acid binding proteins are involved in cellular transports and in the regulation of the solubility of fatty acids <ref> Chmurzyńska A (March 2006). "The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism".''Journal of Applied Genetics''.'''47'''(1): 39-48.https://link.springer.com/article/10.1007/BF03194597</ref>. The biggest difference between the human psoriasin and the rest of the S100 proteins is that the EF-hand at the N-Terminus does not have the ability to bind the calcium <ref> Brodersen DE, Etzerodt M, Madsen P, Celis JE, Thøgersen HC, Nyborg J, Kjeldgaard M (April 1998). "EF-hands at atomic resolution: the structure of human psoriasin(S100A7) solved by MAD phasing". ''Structure''.'''6''': 477-489.https://www.cell.com/structure/pdf/S0969-2126(98)00049-5.pdf</ref>. S100A7 also acts as an anti-microbial protein and it is mainly directed against [https://fr.wikipedia.org/wiki/Escherichia_coli E.Coli]. <ref>Murray J, Boulanger M (April 2010). "S100A7 (S100 calcium binding protein A7)". ''Atlas of Genetics and Cytogeneticsin Oncology and Haematology''. '''15'''(1): 59-64. : http://AtlasGeneticsOncology.org/Genes/S100A7ID42194ch1q21.html</ref>. | ||
== Disease == | == Disease == | ||
| - | == | + | Some S100 proteins such as S100A7 are clearly overexpressed in psoriasis, wound healing, skin cancer, inflammation, cellular stress and other skin conditions. |
| + | Human psoriasin is highly expressed in hyperproliferative skin conditions such as [https://en.wikipedia.org/wiki/Atopic_dermatitis Atopic_dermatitis] and [https://en.wikipedia.org/wiki/Psoriasis Psoriasis] <ref> S Alowami, G Qing, E Emberley, L Snell & PH Watson (2003). "Psoriasin , (S100A7) expression is altered during skin tumorigenesis" ''BMC Dermatology''.'''3'''(1): </ref> which causes a disruption of the skin barrier. In those conditions, scaling occure frequently on joints, like elbows and knees. Psoriasis is a genetic disease that disrupts cell division in skin cells. In a normal epidermal strcucture, [https://en.wikipedia.org/wiki/Keratinocyte keratinocytes] have a regulated process of differentiation. In psoriasis, this differentiation process is disturbed. S100A7 interracts with various signalling proteins and promotes the [https://en.wikipedia.org/wiki/Endothelium endothelial cells] proliferation. The exact role of the protein in psoriasis has not been found yet but its overexpression is thought to be a cause of the perturbation of the differentiation process of keratinocytes. <ref> AK Ekman, J Vegfors, C Bivik Eding, C Enerbäck (December 2016). "Overexpression of Psoriasin (S100A7) Contributes to Dysregulated Differentiation in Psoriasis", ''Acta Derm Venereol''. '''97''': https://www.medicaljournals.se/acta/content/html/10.2340/00015555-2596 </ref> | ||
| + | The study of S100A7 as well as all of the S100 proteins is therefore of great interest in the search for treatments for epidermal diseases. | ||
| + | |||
| + | == Antimicrobial Effect == | ||
| + | |||
| + | Human psoriasin can be used as antimicrobial petide against E-coli. Some mutant of S100A7 are capable of decrease the survival rate of E. coli mainly. Mutation of the conserved carboxyl-terminal EF-hand calcium-binding motif or heat denaturation slightly reduces S100A7 antibacterial activity. Because it modify the structure of the protein and reduce the affinity with others molecules <ref>KC Lee , RL Eckert (April 2007). "S100A7 (Psoriasin) – Mechanism of Antibacterial Action in Wounds", ''Journal of Investigative Dermatology'' '''127'''(4): 945-957 https://www.sciencedirect.com/science/article/pii/S0022202X15333145 </ref>. | ||
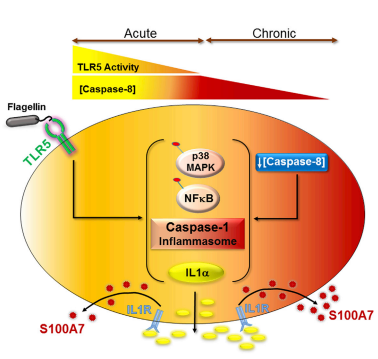
| + | This petide is actually regulated by Caspase-8 using a downregulation of Caspace-8 : mediator of the transcription of the S100A7. This regulation means that less Capase-8 we have more antimicrobial peptide we will get <ref> T Bhatt, A Bhosale, B Bajantri, MS Mathapathi, A Rizvi, G Scita, A Majumdar and C Jamora (November 2019) "Sustained Secretion of the Antimicrobial Peptide S100A7 Is Dependent on the Downregulation of Caspase-8", ''Cell Reports''. '''29'''(9): 2546-2555 https://doi.org/10.1016/j.celrep.2019.10.090 </ref>. [[Image:Caspase8.PNG]] | ||
| - | = | + | The region between the animo acid <scene name='82/829364/The_amino_acid/1'>35-80</scene> is necessery to have a the full antimicrobial effect this amino acid is the central region of S100A7 protein. The others part can be remove without any differencies on the antimicrobial effect. |
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| This Sandbox is Reserved from 25/11/2019, through 30/9/2020 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1091 through Sandbox Reserved 1115. |
To get started:
More help: Help:Editing |
1 PSR
| |||||||||||
References
- ↑ Sedaghat F, Notopoulos A (October 2008). "S100 protein family and its application in clinical practice". Hippokratia. 4: 198-204. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2580040/pdf/hippokratia-12-198.pdf
- ↑ Eckert R, Broome AM, Ruse M, Robinson N, Ryan D, Lee K (July 2004). "S100 Proteins in the Epidermis". Journal of Investigative Dermatology. 123(1): 23-33.https://doi.org/10.1111/j.0022-202X.2004.22719.x
- ↑ Hagens G, Masouyé I, Augsburger E, Hotz R, Saurat JH, Siegenthaler G (April 1999) "Calcium-binding protein S100A7 and epidermal-type fatty acid-binding protein are associated in the cytosol of human keratinocytes" Biochem J. 339(2): 419-427: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1220173/
- ↑ Chmurzyńska A (March 2006). "The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism".Journal of Applied Genetics.47(1): 39-48.https://link.springer.com/article/10.1007/BF03194597
- ↑ Brodersen DE, Etzerodt M, Madsen P, Celis JE, Thøgersen HC, Nyborg J, Kjeldgaard M (April 1998). "EF-hands at atomic resolution: the structure of human psoriasin(S100A7) solved by MAD phasing". Structure.6: 477-489.https://www.cell.com/structure/pdf/S0969-2126(98)00049-5.pdf
- ↑ Murray J, Boulanger M (April 2010). "S100A7 (S100 calcium binding protein A7)". Atlas of Genetics and Cytogeneticsin Oncology and Haematology. 15(1): 59-64. : http://AtlasGeneticsOncology.org/Genes/S100A7ID42194ch1q21.html
- ↑ S Alowami, G Qing, E Emberley, L Snell & PH Watson (2003). "Psoriasin , (S100A7) expression is altered during skin tumorigenesis" BMC Dermatology.3(1):
- ↑ AK Ekman, J Vegfors, C Bivik Eding, C Enerbäck (December 2016). "Overexpression of Psoriasin (S100A7) Contributes to Dysregulated Differentiation in Psoriasis", Acta Derm Venereol. 97: https://www.medicaljournals.se/acta/content/html/10.2340/00015555-2596
- ↑ KC Lee , RL Eckert (April 2007). "S100A7 (Psoriasin) – Mechanism of Antibacterial Action in Wounds", Journal of Investigative Dermatology 127(4): 945-957 https://www.sciencedirect.com/science/article/pii/S0022202X15333145
- ↑ T Bhatt, A Bhosale, B Bajantri, MS Mathapathi, A Rizvi, G Scita, A Majumdar and C Jamora (November 2019) "Sustained Secretion of the Antimicrobial Peptide S100A7 Is Dependent on the Downregulation of Caspase-8", Cell Reports. 29(9): 2546-2555 https://doi.org/10.1016/j.celrep.2019.10.090