Sandbox Reserved 1613
From Proteopedia
(Difference between revisions)
| (71 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
<StructureSection load='6ffc' size='350' frame='true' side='right' caption='Figure 1: ABCG2 6FFC' scene=’’> | <StructureSection load='6ffc' size='350' frame='true' side='right' caption='Figure 1: ABCG2 6FFC' scene=’’> | ||
==Introduction== | ==Introduction== | ||
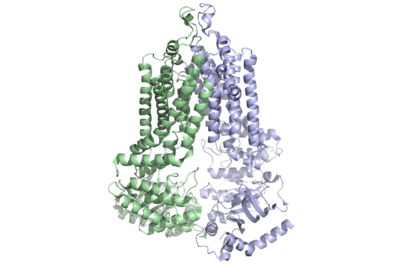
| - | The ABCG2 transporter protein is a notable transmembrane protein | + | [[Image:ABCG2 DimerPic NoWatermark.png|400 px|right|thumb|Figure 1: Multidrug-transporter ABCG2 is a dimer (6ffc).]] |
| + | The ABCG2 transporter protein is a notable [https://en.wikipedia.org/wiki/Transmembrane_protein transmembrane protein]. It transports [https://en.wikipedia.org/wiki/Xenobiotic xenobiotic] material out of cells in many tissues. ABCG2 belongs to the family of 48 transporter proteins called ATP-binding cassette transporters (ABC transporters). The ABC transporters differ from each other by their size structure, and ordering of domains. Ample evidence has shown a link between [https://en.wikipedia.org/wiki/Multiple_drug_resistance multi-drug resistance] and the presence of ABC transporters in the plasma membrane of cells. This is important as multi-drug resistance is one of the major indicators of bad prognoses in cancer treatment. In fact, 19 of the 48 transporters of the ABC family have been shown to transport [https://en.wikipedia.org/wiki/List_of_chemotherapeutic_agents chemotherapeutic agents] out of cells.<ref name="Robey">PMID:29643473</ref> In recent studies, with [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryogenic electronic microscopy] (Cryo EM), the unique <scene name='83/832939/2_cavities/1'>two cavity substrate transport structure</scene>, inward facing nucleotide binding domain and <scene name='83/832939/El-3/3'>condensed EL-3 structure</scene> of ABCG2 have been elucidated, among other features. These new discoveries have allowed for progress towards discovering the exact link between cancer and the ABC transporter family and have allowed for more effective drug treatment of cancer. This page will focus on the family of ABC transporters before delving into unique structural features of ABCG2 and finally describing the effects of this transporter on anti-cancer treatment. | ||
| + | |||
| + | ==ABC Transporter Family== | ||
| + | In the 1990's, [https://en.wikipedia.org/wiki/ATP-binding_cassette_transporter ABC binding cassette transporters] became the subject of much discussion as many were found to have links to the inhibition of [https://en.wikipedia.org/wiki/Molecular_diffusion anti-cancer therapies]. All 48 members of the family were studied and several structural aspects were found to be important to the characterization of [https://en.wikipedia.org/wiki/Transport_protein transporters] in this family. The first was the presence of two [https://en.wikipedia.org/wiki/Nucleotide nucleotide] binding [https://en.wikipedia.org/wiki/Protein_domain domains] (NBD) located in the cytoplasm of all cells which bound and [https://en.wikipedia.org/wiki/Hydrolysis hydrolyzed] [https://en.wikipedia.org/wiki/Adenosine_triphosphate ATP], providing the necessary energy for [https://en.wikipedia.org/wiki/Membrane_transport transport] of the [https://en.wikipedia.org/wiki/Substrate_(chemistry) substrate] to occur. In all 7 subfamilies (A-G) of the ABC family, the NBD's are greatly conserved.<ref name="Robey">PMID:29643473</ref> Each transporter of this family is made unique by the structure and form of their specific transmembrane binding domain (TMD). Each of the 48 transporters have 2 transmembrane domains which work to recognize and transport the substrates across the [https://en.wikipedia.org/wiki/Cell_membrane plasma membrane] and out of the cell. The [https://en.wikipedia.org/wiki/Amino_acid residues] in the TMD provide the transporters each with specific substrates which they can transport. They also allow for the coupling of transport with [https://en.wikipedia.org/wiki/ATP_hydrolysis ATP hydrolysis] to transport molecules regardless of the [https://en.wikipedia.org/wiki/Molecular_diffusion concentration gradient]. | ||
| + | ===Specific Members of the Family=== | ||
| + | As previously mentioned, 19 of the 48 members of the transporter family are involved in chemotherapeutic removal from the cell. Of these, three, <scene name='83/832939/Abcb1/1'>ABCB1</scene>, ABCG2 and <scene name='83/832939/Abcc1/1'>ABCC1</scene> were identified for further study and comparison of [https://en.wikipedia.org/wiki/Chemical_structure structure] due to their function as multi-drug transporters.<ref name="Robey">PMID:29643473</ref> The differences in their structures provided valuable information for scientific research into their substrate binding processes. | ||
| + | |||
==General Structure== | ==General Structure== | ||
| - | The ABCG2 protein is comprised of a homodimer which each have two specific domains: one spanning the cell membrane and one involved with nucleotide binding. | + | The ABCG2 protein is comprised of a [https://en.wikipedia.org/wiki/Molecular_diffusion homodimer] which each have two specific domains: one spanning the cell membrane and one involved with nucleotide [https://en.wikipedia.org/wiki/Ligand_(biochemistry) binding]. |
| - | === | + | === Mechanism of Substrate Transport === |
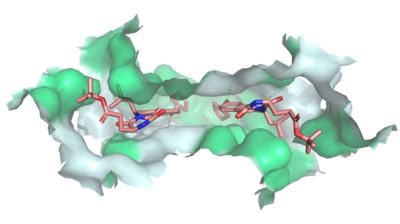
| - | + | [[Image:Ligand_Interactions_6ffc.png|400 px|right|thumb|Figure 2: MZ29 bound to cavity 1 of ABCG2 (6ffc). Two MZ29 are shown in sticks and are colored by element. Hydrophobic interactions between the surface of cavity 1 and MZ29 are shown in green.]] | |
| - | + | ||
| - | + | Multidrug Transporter ABCG2 is a <scene name='83/832937/Dimer/1'>dimer</scene> that consists of two [https://en.wikipedia.org/wiki/Cavity cavities] separated by a <scene name='83/832937/Leucine_plug/4'>leucine plug</scene>. Cavity 1 is a binding pocket open to the [https://en.wikipedia.org/wiki/Cytoplasm cytoplasm] and the inner leaflet of the plasma membrane. Its shape is suitable to bind flat, hydrophobic and polycyclic substrates.<ref name="Manolaridis">PMID:30405239</ref> Many of its amino acids residues form hydrophobic interactions with the bound substrate, as shown in green in '''Figure 1'''. Cavity 2 is located above the leucine plug. It is empty until a <scene name='83/832937/Atp_and_mg_bound_to_abcg2/4'>magnesium ion and ATP</scene> are bound to ABCG2.<ref name="Taylor">PMID:28554189</ref> Its <scene name='83/832937/Cysteine_disulfide_bridges/5'>inter- and intra-disulfides</scene> (yellow is inter- and intra-molecular disulfides, golden is intra-molecular only) promote the release of the substrate from the cavity into the extracellular space. | |
| + | One interesting feature of the NBD's is the fact that they remain in contact with one another even without a bound substrate. This makes the ABCG2 transporter unique and provides greater substrate specificity as the entrance to the transporter is not as globular as either ABCB1 or ABCC1. The entrance from the cytoplasm to the transporter is a [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] membrane entrance lined by <scene name='83/832939/Lining_of_entrance_of_nbd/1'>residues A397, V401, L405, L539, I543 and T547</scene> in both [https://en.wikipedia.org/wiki/Monomer monomers]. | ||
| + | Dimerization of ABCG2 was originally thought to be achieved with the help of the <scene name='83/832939/Disproved_dimerization_process/1'>406xxx410 structural motif</scene> in each of the two domains but Cryo-EM showed that the [https://en.wikipedia.org/wiki/Sequence_motif motifs] were on opposite sides of the protein.<ref name="Manolaridis" /> <ref name="Jackson">PMID:29610494</ref> | ||
| + | |||
| + | |||
| + | ===Transmembrane Domain Stabilization=== | ||
| + | In the transmembrane domain is located the leucine plug that separates the first site of binding from the second site of binding for the substrate. Both domains are stabilized by several interactions. The first and most prominent feature of the TMD is EL-3. This loop which extends out from the transmembrane domain has been shown to be involved in stabilization of the TMD's. A member of the same [https://en.wikipedia.org/wiki/Subfamily subfamily], ABCG5/ABCG8, was shown to have <scene name='83/832939/El-3_of_abcg5_abcg8/1'>EL-3 helices</scene> that extended further into the extracellular space. This condensed helices is one of the defining features of ABCG2 transporter protein. The EL-3 of ABCG2 is stabilized by both intermolecular and intramolecular [https://en.wikipedia.org/wiki/Disulfide disulfide bonds] at <scene name='83/832939/Disulfide_bonding_interactions/1'>592, 608 and between Cys 603</scene> of each domain. ABCG8/ABCG5 do not have disulfide bonds present in EL-3 making it possible that intramolecular disulfide bonds in ABCG2 play a part in multiple drug transport as ABCG5/ABCG8 does not transport multiple chemotherapeutic drugs and has much less promiscuity of substrates than ABCG2. Even with the stabilization of the disulfide bonds, it has been discovered that without [https://en.wikipedia.org/wiki/Glycosylation glycosylation] at <scene name='83/832939/6ffc-glycosylation/1'>N 596</scene>, ABCG2 does not mature and is [https://en.wikipedia.org/wiki/Ubiquitin ubiquitin] tagged for degradation. | ||
| + | |||
==Function== | ==Function== | ||
| - | ABCG2 transports a variety of <scene name='83/832939/Mz29/1'>substrates</scene>, particularly flat, hydrophobic, and/or | + | ABCG2 transports a variety of <scene name='83/832939/Mz29/1'>substrates</scene>, particularly flat, hydrophobic, and/or polycylcic molecules.<ref name="Jackson">PMID:29610494</ref> ABCG2 is found in multiple biological membranes, especially at blood barriers such as the [https://en.wikipedia.org/wiki/Blood brain barrier], [https://en.wikipedia.org/wiki/Blood testis barrier], and the blood-placental barrier. The prevalence of ABCG2 protects those tissues from cytotoxins. In addition to cytotoxin protection, ABCG2 secretes endogenous substrates in the adrenal gland, excretes toxins in the liver and kidneys, and regulates absorption of substrates.<ref name="Fetsch">PMID:15990223</ref> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | == | + | ===Fab-5D3=== |
| - | + | In the mid 2010's, the use of 2 antigen binding fragments <scene name='83/832939/Abcg2_with_bound_5d3-fab/4'>(5D3-Fab)</scene> allowed for high resolution images to finally be developed for ABCG2 transporter protein. With these recent discoveries, the understanding of this protein has greatly increased in the last several years. Before these images, ABCG2 had been unable to be recorded at high resolution as the domains were always moving. 5D3 Fab <scene name='83/832939/Fab_binding_site/2'>clamps</scene> the two domains together preventing movement of the transporter from inward to outward facing. Fab binds at a 35 degree angle relative to the membrane plane which stops the 40 degree transition of the TMD from closed to open. Interestingly, complete arrest of ABCG2 in its inward facing state only requires one Fab bound but in experiments, two Fabs bound, each to both domains. | |
| - | + | ||
| - | + | ||
| - | + | ===Polymorphic Changes=== | |
| - | + | Several mutations have been shown to also decrease transporter activity. The first and most important of these is a point mutation of Glu 211 to Gln 211. This <scene name='83/832939/Residue_211/1'>mutation</scene> completely abolished activity of the transporter providing evidence of a significant role in the transport of substrates. Another important point mutation occurs at at <scene name='83/832939/The_cause_of_gout/1'>Q141</scene>, which becomes K141 and is the cause of gout. This mutation causes distortions of the protein's tertiary structure. The last mutation of note is at <scene name='83/832939/R482/1'>R482</scene>. Changes at this residue has been shown to change substrate specificity but as it is not in the binding pocket, this residue achieves this by allosteric interactions. | |
| - | + | ||
| + | == Disease == | ||
| + | One of the causes for multidrug resistant cancers is the excretion of cancer drugs out of the cell, thereby decreasing the effective intracellular concentration. ABCG2, also known as the breast cancer resistance protein (BCRP), effluxes multiple chemotherapeutic agents such as [https://en.wikipedia.org/wiki/Mitoxantrone mitoxantrone] and [https://en.wikipedia.org/wiki/Camptothecin camptothecin] analogies, making the cancerous breast cells resistant to chemotherapy. Competitive inhibitors, such as <scene name='83/832939/Abcg2_bound_to_mz29/3'>MZ29</scene>, shut down ABCG2 to stop the efflux of cancer drugs in order to combat the resistivity of breast cancer. <ref name="Jackson" /> | ||
| + | |||
| + | The ABC transporter family has been found as a prevalent piece of multi-drug resistant cancers and therefore became a popular target towards inhibition. Three generations of drugs were made in order to inhibit a similar protein from the same family, ABCC1 at its interior binding site including cyclosporine A (first generation), valspodar (second generation), and Elacridar (3rd generation). Importantly, cyclosporine A and Elacridar were found to inhibit both ABCC1 and ABCG2 and in one trial had success along with chemotherapy in the treatment of acute myeloid leukemia but because of either side effects or experimentation that was not able to be duplicated, this research was mostly shelved. The main issue in their failure to find a drug to inhibit this protein was the failure to develop a high-resolution structure of this protein with the technology available at the time of this drug development. Now, with high resolution images of the structures, fourth generation inhibitors are being developed with more success in inhibition without additional drug interactions or cell toxicity. | ||
| + | == Relevance == | ||
| + | As previously stated, by utilizing certain binders(<scene name='83/832939/5d3-fab/2'>5D3</scene>), it is able to be stabilized for crystallographic imaging.<ref name="Taylor">PMID:28554189</ref> This has allowed researchers in the past decade to make advances based upon the greater understanding of its structure. Using these advances, inhibitors have been found to stop effluxion by ABCG2. Completely inhibiting this function, however, has residual effects on the excretory system. One such effect is decreased uric acid excretion in both the kidneys and the gut, which causes [https://en.wikipedia.org/wiki/Hyperuricemia hyperuricemia] . This results in an increased risk of uric acid crystal build-up, known as [https://en.wikipedia.org/wiki/Tophus tophi formation], which causes a type of arthritis known as [https://en.wikipedia.org/wiki/Gout gout] . Balancing the inhibition of ABCG2 will determine how to lessen these effects while continuing to combat cancer resistivity.<ref name="Cleophas">PMID:28461764</ref> | ||
</StructureSection> | </StructureSection> | ||
==References== | ==References== | ||
| - | <ref name=”Jackson”>PMID:29610494</ref> | ||
| - | <ref name="Manolaridis">PMID:30405239</ref> | ||
| - | <ref name="Taylor">PMID:28554189</ref> | ||
| - | <ref name="Fetsch">PMID:15990223</ref> | ||
<References/> | <References/> | ||
==Student Contributors== | ==Student Contributors== | ||
| + | Shelby Skaggs, | ||
Samuel Sullivan, | Samuel Sullivan, | ||
| - | Jaelyn Voyles | + | Jaelyn Voyles |
| - | + | ||
Current revision
ABCG2 Transporter Protein
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018 Jul;18(7):452-464. doi: 10.1038/s41568-018-0005-8. PMID:29643473 doi:http://dx.doi.org/10.1038/s41568-018-0005-8
- ↑ 2.0 2.1 Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
- ↑ 3.0 3.1 Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
- ↑ 4.0 4.1 4.2 Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
- ↑ Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, Bates SE. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006 Apr 8;235(1):84-92. doi: 10.1016/j.canlet.2005.04.024. Epub, 2005 Jun 28. PMID:15990223 doi:http://dx.doi.org/10.1016/j.canlet.2005.04.024
- ↑ Cleophas MC, Joosten LA, Stamp LK, Dalbeth N, Woodward OM, Merriman TR. ABCG2 polymorphisms in gout: insights into disease susceptibility and treatment approaches. Pharmgenomics Pers Med. 2017 Apr 20;10:129-142. doi: 10.2147/PGPM.S105854., eCollection 2017. PMID:28461764 doi:http://dx.doi.org/10.2147/PGPM.S105854
Student Contributors
Shelby Skaggs, Samuel Sullivan, Jaelyn Voyles