This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1639
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 15: | Line 15: | ||
== Important amino acids == | == Important amino acids == | ||
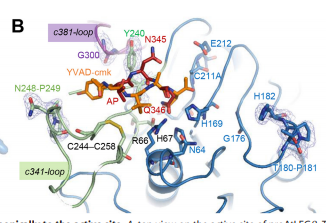
| - | To dig deeper and better understand the protein, you can see where the <scene name='86/861621/Protein_view_2/3'>ligand is bound to the protein</scene>. However, these ligands aren't entirely that important to the active site. The ligands shown are there to help stabilize the structure. The actual important enzyme of the active site is actually the activation peptide(AP). This AP [[Image:AP.PNG]] is made up of amino acid residues of ASN345 (asparagine), GLN346 (glutamine), ARG347 (arginine), which form<scene name='86/861621/Catalytic_triad/1'> the catalytic triad</scene> and where the cleavage happens. This cleavage basically splits the sequence in half of prime and nonprime on either side of the split. Then, the split sequences then take on a cyclic formation. | + | To dig deeper and better understand the protein, you can see where the <scene name='86/861621/Protein_view_2/3'>ligand is bound to the protein</scene>. However, these ligands aren't entirely that important to the active site. The ligands shown are there to help stabilize the structure. The actual important enzyme of the active site is actually the activation peptide(AP). This AP [[Image:AP.PNG]] is made up of amino acid residues of ASN345 (asparagine), GLN346 (glutamine), ARG347 (arginine), which form<scene name='86/861621/Catalytic_triad/1'> the catalytic triad</scene> and where the cleavage happens. This cleavage [[Image:Cleavage.png]] basically splits the sequence in half of prime and nonprime on either side of the split. Then, the split sequences then take on a cyclic formation. This cyclic formation is crucial for the protein to remain in homeostasis and is the main source of energy transformation with bonds being broken and recreated during cyclization. |
Our protein is also very <scene name='86/861621/Hydrophobic_parts/1'>hydrophobic at certain ends.</scene> [[Image:Hydrophobic.png]] | Our protein is also very <scene name='86/861621/Hydrophobic_parts/1'>hydrophobic at certain ends.</scene> [[Image:Hydrophobic.png]] | ||
== Structural highlights == | == Structural highlights == | ||
| Line 25: | Line 25: | ||
== Other important features == | == Other important features == | ||
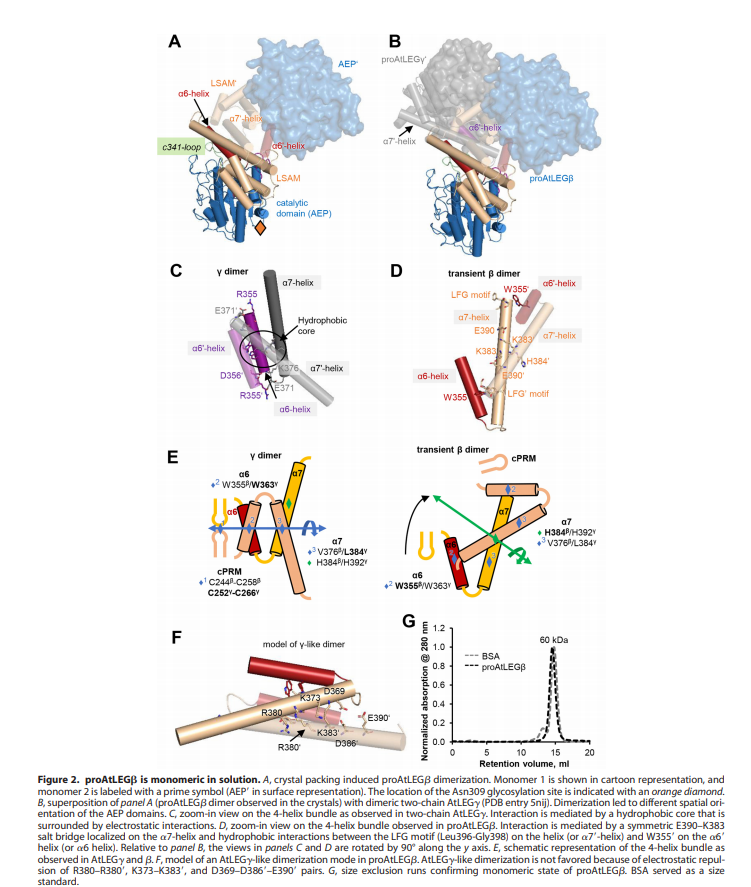
| - | An important feature is that proAtLEGb forms atypical dimers in the crustal and is monomeric in solutions. When compared to another form of our protein AtLEGgy findings suggest that the observed beta-dimer is weak and probably only transient in solution. | + | An important feature is that proAtLEGb forms atypical dimers in the crustal and is monomeric in solutions. When compared to another form of our protein AtLEGgy findings suggest that the observed beta-dimer is weak and probably only transient in solution. [[Image:Monomeric.png]] |
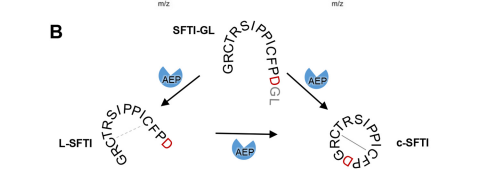
Another important feature is the cyclization of the peptides after cleavage. The cyclization is important for the cell stays programmed and is the same every time. [[Image:Cyclization.png]] | Another important feature is the cyclization of the peptides after cleavage. The cyclization is important for the cell stays programmed and is the same every time. [[Image:Cyclization.png]] | ||
Current revision
| This Sandbox is Reserved from 09/18/2020 through 03/20/2021 for use in CHEM 351 Biochemistry taught by Bonnie Hall at Grand View University, Des Moines, IA. This reservation includes Sandbox Reserved 1628 through Sandbox Reserved 1642. |
To get started:
More help: Help:Editing |
Arabidopsis thaliana legumain isoform beta in zymogen state (6YSA)
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
[1] PMID:32719006