We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox GGC15
From Proteopedia
(Difference between revisions)
| (45 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | == | + | ==DNA TOPOISOMERASE I== |
| - | <StructureSection load=' | + | <StructureSection load='1A36' size='340' side='right' caption='Caption for this structure' scene=''> |
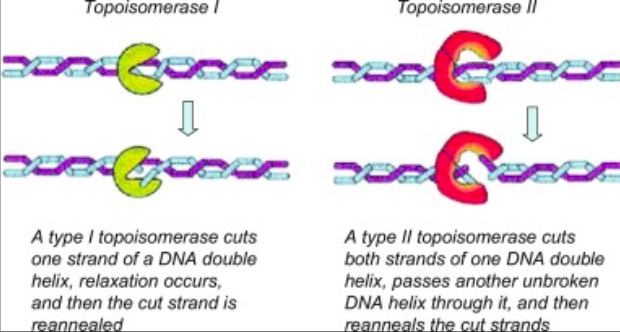
| - | + | '''Eukaryotic DNA topoisomerase I (topo I) is a protein that reduces the strain from the supercoils that are caused during transcription and translation<ref name="Staker">DOI 10.1073/pnas.242259599</ref>. There are two types of topoisomerases. Type 1 topoisomerases are monomeric and break one strand of DNA<ref name="Redinbo">PMID:9488644</ref>. Type 2 topoisomerases are dimeric, meaning that they made up of two units and break both strands of the DNA helix<ref name="Redinbo" />. They are able to pass another part of the duplex through the cut, and close the cut using ATP<ref name="Staker" />. | |
| - | + | [[Image:04_27_21_A136_Top_1_and_Top_2_Example.jpg]]. <ref name="Dyakonov">D'yakonov, V. A., Dzhemileva, L. U., & Dzhemilev, U. M. (2017). Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Studies in Natural Products Chemistry, 21–86. https://doi.org/10.1016/b978-0-444-63929-5.00002-4 </ref>.''' | |
| + | |||
| + | == Structure == | ||
| + | '''Human topo 1 is composed of 765 amino acids <ref name="Redinbo" />. The enzyme consist of 4 regions which are the NH2-terminal, core, linker, and COOH-terminal domains<ref name="Redinbo" />. The NH2-terminal is approximately 210 residues long, it is highly charged, disordered, and contains few hydrophobic amino acids<ref name="Redinbo" />. The <scene name='78/781215/04_27_structure_cooh/1'>COOH-terminal</scene> domain is made up of residues 713 to 765 and contains the important amino acid <scene name='78/781215/04_27_structure_cooh_with_k/1'>Tyrosine 723</scene><ref name="Redinbo"/>. The location of the active site is at this amino acid<ref name="Redinbo" />. Residues 636 to 712 form the <scene name='78/781215/04_27_structure_linker/1'>Linker domain</scene> and they contribute to the enzyme catalytic activity but are not required<ref name="Redinbo" />. The core is divided into 3 subdomains: <scene name='78/781215/04_27_structure_core_sd1/1'>Core Sudomain 1</scene>[215-232 & 320-433], <scene name='78/781215/04_27_structure_core_sd2/1'>Core Subdomain 2</scene>[233-319], and <scene name='78/781215/04_27_structure_core_sd3/1'>Core Subdomain 3</scene>[434-635]<ref name="Redinbo"/>. The <scene name='78/781215/04_27_structure_core_cooh/1'>core and the COOH-terminal domains</scene> are very important for the catalytic activity<ref name="Redinbo" />.''' | ||
| + | [[Image:Color Key.jpg]] | ||
| + | == Active Site & Mechanism Of Action== | ||
| + | '''Topo 1 reduces stress in DNA by causing a transient single strand nick in the the DNA helix<ref name="Staker" />. This nick enables the cut to rotate around its intact complement, thus eliminating proximal supercoils<ref name="Staker" />.''' | ||
| + | |||
| + | |||
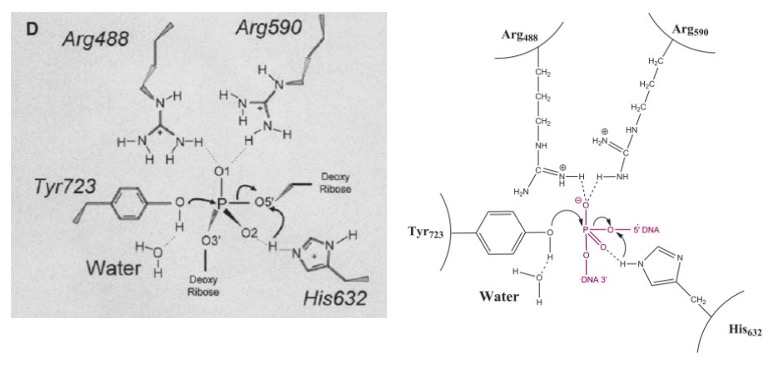
| + | '''The active site of Topo 1 is catalytic and it is the location where the nicking or cutting occurs<ref name="Redinbo" />. The nicking occurs from the trans-esterification of Tyr-723 at a DNA phophodiester bond forming a <scene name='78/781215/04_27_19_active_site_mech/1'>3'-phosphotyrosine covalent enzyme–DNA complex</scene> <ref name="Staker" />. After the DNA is relaxed, the covalent intermediate is reversed when the released 5'-OH of the broken strand reattacks the phosphotyrosine intermediate in a second transesterification reaction<ref name="Staker" />.''' | ||
| + | |||
| + | [[Image:Active_Site_3_PM.jpg]]<ref name ="Stewart">Stewart, L. (1998). A Model for the Mechanism of Human Topoisomerase I. Science, 279(5356), 1534–1541. https://doi.org/10.1126/science.279.5356.1534</ref> | ||
| + | |||
| + | [[Image:DNA and Tyrosine.jpg]]<ref name="Stewart" /> | ||
| - | == Function == | ||
| - | == Disease == | ||
== Relevance == | == Relevance == | ||
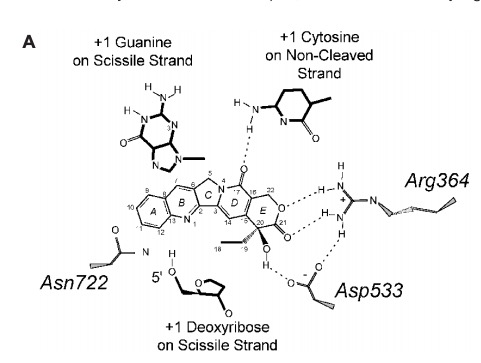
| + | '''Many anticancer drugs target topo 1 enzymes. This enzyme is the target of camptothecin (CPT) family of anticancer drugs<ref name="Redinbo" />. These drugs work by increasing the duration of the nicked intermediate in the <scene name='78/781215/04_28_revelance_cpt_bind/1'>topo I</scene> reaction <ref name="Redinbo" />. CPT enhances DNA breakage at sites with a guanine base at the +1 position on the DNA strand that is being cut<ref name="Redinbo" />. This is immediately downstream of the site that is being cleaved<ref name="Redinbo" />. The stabilized intermediates prevent transcription and replication to continue in the cancer cells<ref name="Redinbo" />. This eventually leads to DNA damage and cell death<ref name="Redinbo" />.''' | ||
| + | [[Image:CPT and Enzyme.jpg]] <ref name="Redinbo" /> | ||
| + | |||
| + | == Mutations == | ||
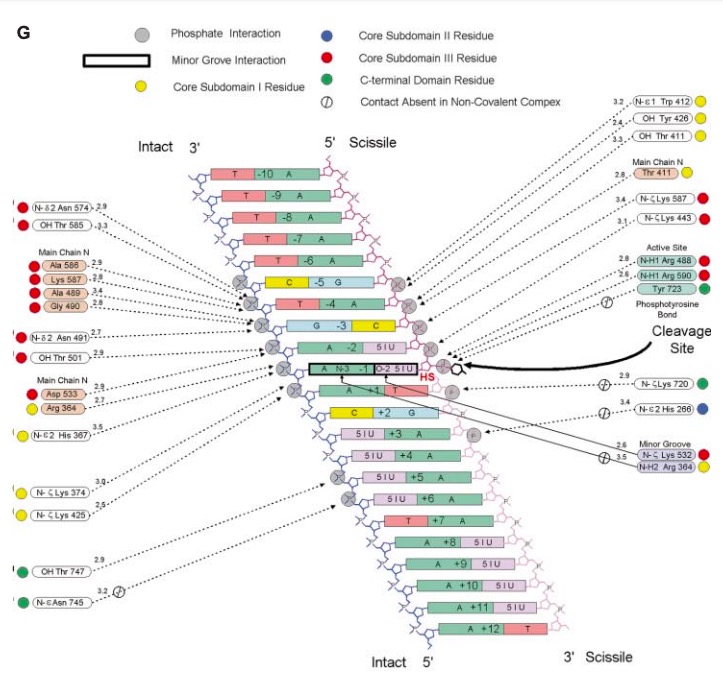
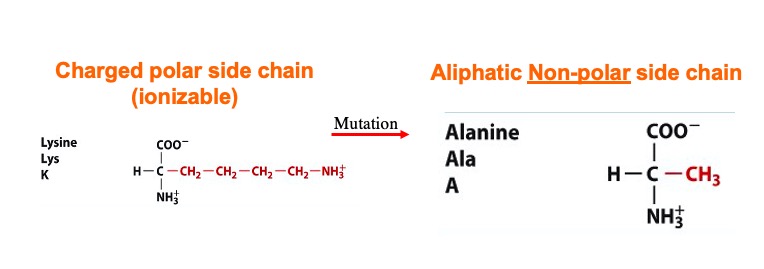
| + | '''A mutation at amino acid <scene name='78/781215/04_27_19_mutation_1/1'>Lysine</scene> 532 to Alanine almost abolishes enzyme activity<ref name="Interthal">DOI 10.1074/jbc.M309959200</ref>. The location of Lys 532 to the scissile phosphate and other active site amino acids could be the reason why a mutation of this amino acid abolishes the enzyme activity<ref name="Interthal" />. | ||
| - | + | [[Image:Screen_Shot_2021-04-27_at_7.28.18_PM.jpg]]''' | |
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
DNA TOPOISOMERASE I
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15387-92. Epub 2002 Nov 8. PMID:12426403 doi:10.1073/pnas.242259599
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WG. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science. 1998 Mar 6;279(5356):1504-13. PMID:9488644
- ↑ D'yakonov, V. A., Dzhemileva, L. U., & Dzhemilev, U. M. (2017). Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Studies in Natural Products Chemistry, 21–86. https://doi.org/10.1016/b978-0-444-63929-5.00002-4
- ↑ 4.0 4.1 Stewart, L. (1998). A Model for the Mechanism of Human Topoisomerase I. Science, 279(5356), 1534–1541. https://doi.org/10.1126/science.279.5356.1534
- ↑ 5.0 5.1 Interthal H, Quigley PM, Hol WG, Champoux JJ. The role of lysine 532 in the catalytic mechanism of human topoisomerase I. J Biol Chem. 2004 Jan 23;279(4):2984-92. Epub 2003 Oct 31. PMID:14594810 doi:10.1074/jbc.M309959200