We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox testing Sn2 for Veronika
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==Your Heading Here (maybe something like 'Structure')== | ==Your Heading Here (maybe something like 'Structure')== | ||
<StructureSection load='Rot_Sn2.xyz' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='Rot_Sn2.xyz' size='340' side='right' caption='Caption for this structure' scene=''> | ||
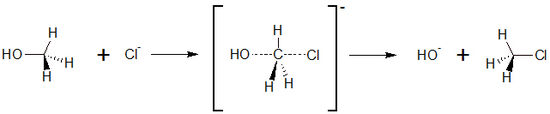
| - | + | SN2 reaction is a basic reaction type in organic chemistry. The letter S<sub>N</sub> stands for nulceophilic Substitution, the number 2 stands for bimolecular, with both reactions partners are involved in the reaction rate determining step. It also exists an S<sub>N</sub>1 reaction; here, only one reaction partner is involved in this step. On the other side, SN2 reactions are characterized by exchanging substituents. The substituent that leaves the molecule is called leaving group. | |
| - | + | ||
| + | Typically, alkanes with a substituent in primary position undergo S<sub>N</sub>2 reactions. In contrast to a S<sub>N</sub>1 reaction, no stable carbo cation can be formed, therefore, another way is taken. | ||
| + | The S<sub>N</sub>2 <jmol><jmolLink><script>anim mode once; frame range 1 10; delay 0.5; frame play</script><text>reaction starts</text></jmolLink></jmol> by establishing a so called ''intermediate'' state, with both educts comeing close to each other. By this, the bond of the leaving group is partly broken, and the bond to the new group is partly formed. The formation of this intermediate state is the rate determining step of the reaction. | ||
| + | In the <jmol><jmolLink><script>anim mode once; frame range 11 20; delay 0.5; frame play</script><text>second step</text></jmolLink></jmol>, the bond of the leaving group is completely broken, and at the same time the bond to the new substituent is completely formed. | ||
| + | [[Image:reaction_scheme_sn2.jpg|550px]] <br> | ||
| + | The S<sub>N</sub>2 reaction has a very interesting stereochemistry inversion of the stereocenter<ref>PMID: 27505286 </ref> with an animated example of the substitution of chloride and methanol shown. Please click on the buttons below to '''animate''' the reaction with different representations. Use the '''popup''' button to enlarge the view and the '''quality''' button to turn on anti-aliasing. | ||
| Line 26: | Line 30: | ||
</jmol> | </jmol> | ||
| - | == Function == | ||
| - | + | </StructureSection> | |
| + | The animation was originally done by [[User:Verena Pietzner | Prof. Dr. Verena Pietzner]]; for details, see her web site ChiLe<ref>[http://www.chemieunterricht-interaktiv.de/en/index.html ChiLe Web Site]</ref>. The implementation into Proteopedia was done by [[User:Jaime Prilusky | Prof. Jaime Prilusky]], [[User:Joel L. Sussman | Prof. Joel L. Sussman ]] | ||
| + | and [[User:Veronika Pelekhov | Veronika Pelekhov]]. | ||
| - | == | + | ===See also=== |
| + | [[SN1_reaction|S<sub>N</sub>1 reaction: Substitution of Cl<sup>−</sup> and ''tert''-Butanol ]] | ||
| - | == Structural highlights == | ||
| - | |||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
| - | |||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Your Heading Here (maybe something like 'Structure')
| |||||||||||
The animation was originally done by Prof. Dr. Verena Pietzner; for details, see her web site ChiLe[2]. The implementation into Proteopedia was done by Prof. Jaime Prilusky, Prof. Joel L. Sussman and Veronika Pelekhov.
See also
SN1 reaction: Substitution of Cl− and tert-Butanol
References
- ↑ Wang Y, Song H, Szabo I, Czako G, Guo H, Yang M. Mode-Specific SN2 Reaction Dynamics. J Phys Chem Lett. 2016 Sep 1;7(17):3322-7. doi: 10.1021/acs.jpclett.6b01457. Epub, 2016 Aug 12. PMID:27505286 doi:http://dx.doi.org/10.1021/acs.jpclett.6b01457
- ↑ ChiLe Web Site