User:Mathias Bortoletto Dunker/Sandbox 1
From Proteopedia
< User:Mathias Bortoletto Dunker(Difference between revisions)
| (35 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
<StructureSection load='2Flu' size='340' side='right' caption='Crystal Structure of the Kelch-Neh2 Complex' scene='89/898348/Flu2/1'> | <StructureSection load='2Flu' size='340' side='right' caption='Crystal Structure of the Kelch-Neh2 Complex' scene='89/898348/Flu2/1'> | ||
| - | Keap1 is a BTB-Kelch substrate adaptor protein anchored to the actin cytoskeleton that regulates steady-state levels of [https://en.wikipedia.org/wiki/NFE2L2 Nrf2],<ref name="main">Lo, S.-C., Li, X., Henzl, M.T., Beamer, L.J. and Hannink, M. (2006), Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25: 3605-3617. https://doi.org/10.1038/sj.emboj.7601243 </ref> one of the main transcription factor of cytoprotective genes found in mammals.<ref>Pitoniak, A., & Bohmann, D. (2015). Mechanisms and functions of Nrf2 signaling in Drosophila. Free radical biology & medicine, 88(Pt B), 302–313. https://doi.org/10.1016/j.freeradbiomed.2015.06.020</ref> Under homeostatic conditions, Keap1 is capable of marking Nfr2 for [https://en.wikipedia.org/wiki/Ubiquitin ubiquitin-dependent degradation], repressing the expression of genes associated with cytological defense against highly reactive molecules, such as electrophilic chemicals, heavy metals and oxidative agents. <ref name="main" /> As such, when the cell is subjected to stresses of those kinds, Nrf2 is no longer targeted for ubiquitin-dependent degradation, thus allowing for the expressions of the genes responsible for the cellular defense against such reactive substances. | + | Keap1 is a BTB-Kelch substrate adaptor protein anchored to the actin cytoskeleton that regulates steady-state levels of [https://en.wikipedia.org/wiki/NFE2L2 Nrf2],<ref name="main">Lo, S.-C., Li, X., Henzl, M.T., Beamer, L.J. and Hannink, M. (2006), Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25: 3605-3617. https://doi.org/10.1038/sj.emboj.7601243 </ref> one of the main transcription factor of cytoprotective genes found in mammals.<ref>Pitoniak, A., & Bohmann, D. (2015). Mechanisms and functions of Nrf2 signaling in Drosophila. Free radical biology & medicine, 88(Pt B), 302–313. https://doi.org/10.1016/j.freeradbiomed.2015.06.020</ref> Under homeostatic conditions, <scene name='89/898348/Flu2/1'>Keap1 is capable of marking Nfr2 </scene> for [https://en.wikipedia.org/wiki/Ubiquitin ubiquitin-dependent degradation], repressing the expression of genes associated with cytological defense against highly reactive molecules, such as electrophilic chemicals, heavy metals and oxidative agents. <ref name="main" /> As such, when the cell is subjected to stresses of those kinds, Nrf2 is no longer targeted for ubiquitin-dependent degradation, thus allowing for the expressions of the genes responsible for the cellular defense against such reactive substances. |
{| class="wikitable" | {| class="wikitable" | ||
| Line 36: | Line 36: | ||
|} | |} | ||
| - | == | + | == Mechanism and function of Keap 1 == |
In eukaryote cells, the defense against reactive molecules from both endogenous or exogenous sources are usually coordinated thanks to the expression of genes regulated by the Cap N' Collar transcription factors, a unique subset within the [https://en.wikipedia.org/wiki/BZIP_domain bZIP] family of transcription factors.<ref> Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;(71):157-76. doi: 10.1042/bss0710157. PMID: 15777020. </ref> Such molecules (reactive oxygen species, electrophilic chemicals and heavy metals), if not neutralized and eliminated, can affect the oxidation levels of most endocytic molecules and compromise the integrity of the DNA, leading to severe damage to the organism and causing many pathophysiological processes, including many known diseases in humans such as cancer<ref>Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001 Jun 2;477(1-2):7-21. doi: 10.1016/s0027-5107(01)00091-4. PMID: 11376682. </ref>and cardiovascular problems. <ref>Ceconi C, Boraso A, Cargnoni A, Ferrari R. Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys. 2003 Dec 15;420(2):217-21. doi: 10.1016/j.abb.2003.06.002. PMID: 14654060. </ref> The expression of genes regulated by the Cap N' Collar (CNC) set — which includes the transcription factors Nrf1, Nrf2, Nrf3, Bach1 and Bach2 — are indispensable for the induction of enzymes that can neutralize such reactive molecules, eliminate damaged macromolecules and restore cellular redox homeostasis.<ref name="main" /> | In eukaryote cells, the defense against reactive molecules from both endogenous or exogenous sources are usually coordinated thanks to the expression of genes regulated by the Cap N' Collar transcription factors, a unique subset within the [https://en.wikipedia.org/wiki/BZIP_domain bZIP] family of transcription factors.<ref> Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;(71):157-76. doi: 10.1042/bss0710157. PMID: 15777020. </ref> Such molecules (reactive oxygen species, electrophilic chemicals and heavy metals), if not neutralized and eliminated, can affect the oxidation levels of most endocytic molecules and compromise the integrity of the DNA, leading to severe damage to the organism and causing many pathophysiological processes, including many known diseases in humans such as cancer<ref>Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001 Jun 2;477(1-2):7-21. doi: 10.1016/s0027-5107(01)00091-4. PMID: 11376682. </ref>and cardiovascular problems. <ref>Ceconi C, Boraso A, Cargnoni A, Ferrari R. Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys. 2003 Dec 15;420(2):217-21. doi: 10.1016/j.abb.2003.06.002. PMID: 14654060. </ref> The expression of genes regulated by the Cap N' Collar (CNC) set — which includes the transcription factors Nrf1, Nrf2, Nrf3, Bach1 and Bach2 — are indispensable for the induction of enzymes that can neutralize such reactive molecules, eliminate damaged macromolecules and restore cellular redox homeostasis.<ref name="main" /> | ||
| - | <scene name='89/898348/Crystal_structure_of_the_kelch/1'>Keat1 is a BTB-Kelch</scene> substrate adaptor protein responsible for regulating the levels of Nrf2, which together with Nrf1 constitutes the two most prevalent CNC transcription factors in regards to endocytic protection against reactive species in mammals, according to a 2003 experiment that consisted on the observation of the impact of individual CNC genes deletion.<ref> Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003 Nov 28;278(48):48021-9. doi: 10.1074/jbc.M308439200. Epub 2003 Sep 10. PMID: 12968018.</ref> Under basal conditions, Keap1 targets Nrf2 for ubiquitin-dependent degradation<ref name="main" /> by bridging the Neh2 domain (a conserved N-terminal regulatory domain) of the Nrf2 to a molecule of <scene name='89/898348/Cul3_with_keap1/1'>CUL3</scene>, a component of Cullin-RING E3 ubiquitin ligases complexes (CRLs). Once the lysine residues within the Neh2 domain of Nrf2 are marked, cyclical association and dissociation of this E3 ubiquitin ligase complex, mediated by the opposing actions of CAND1 and Cul3 neddylation, enables efficient ubiquitination of Nrf2<ref name="main" />, repressing the expression of Nrf2 dependent genes, reducing the ability of the cell to neutralize reactive molecules. But when the cell is under oxidative stress, a array of <scene name=' | + | <scene name='89/898348/Crystal_structure_of_the_kelch/1'>Keat1 is a BTB-Kelch</scene> substrate adaptor protein responsible for regulating the levels of Nrf2, which together with Nrf1 constitutes the two most prevalent CNC transcription factors in regards to endocytic protection against reactive species in mammals, according to a 2003 experiment that consisted on the observation of the impact of individual CNC genes deletion.<ref> Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003 Nov 28;278(48):48021-9. doi: 10.1074/jbc.M308439200. Epub 2003 Sep 10. PMID: 12968018.</ref> Under basal conditions, Keap1 targets Nrf2 for ubiquitin-dependent degradation<ref name="main" /> by bridging the Neh2 domain (a conserved N-terminal regulatory domain) of the Nrf2 to a molecule of <scene name='89/898348/Cul3_with_keap1/1'>CUL3</scene>, a component of Cullin-RING E3 ubiquitin ligases complexes (CRLs). Once the lysine residues within the Neh2 domain of Nrf2 are marked, cyclical association and dissociation of this E3 ubiquitin ligase complex, mediated by the opposing actions of CAND1 and Cul3 neddylation, enables efficient ubiquitination of Nrf2<ref name="main" />, repressing the expression of Nrf2 dependent genes, reducing the ability of the cell to neutralize reactive molecules. But when the cell is under oxidative stress, a array of <scene name='89/898348/Keap1micecys/1'>sulfhydryl groups of Keap1</scene> are modified,<ref name="albena">Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11908-13. doi: 10.1073/pnas.172398899. Epub 2002 Aug 22. PMID: 12193649; PMCID: PMC129367.</ref> perturbing the ubiquitin ligase complex and raising the levels of Nrf2, thus allowing for the expression of the genes responsible for the cytological defense against the invasive molecule. Although there are differences between the amino acid sequence of the Keap1 protein present in all eight organisms whose sequences have been stablished (mouse, rat, pig, human, Xenopus, zebrafish, Drosophila and rice)<ref name="res">Albena T. Dinkova-Kostova, W. David Holtzclaw, and Thomas W. Kensler. The Role of Keap1 in Cellular Protective Responses. American Chemical Society, August 2, 2005.</ref>, studies have shown that in mice, the detection of xenobiotic agents is done by intermolecular disulfide bridges present in the Cys273 and Cys288 residues of the IVR domain, probably between C273 of one Keap1 molecule and C288 of a second<ref>Nobunao Wakabayashi, Albena T. Dinkova-Kostova, W. David Holtzclaw, Moon-Il Kang, Akira Kobayashi, Masayuki Yamamoto, Thomas W. Kensler, and Paul Talalay. Protection against electrophile and oxidant stress by |
| + | induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. PNAS February 17, 2004 vol. 101 no. 7.</ref>. | ||
| + | |||
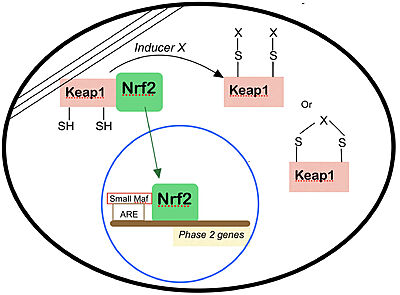
[[Image:Nrf2.jpg|400px|center|]] | [[Image:Nrf2.jpg|400px|center|]] | ||
Nrf2 is anchored in the cytoplasm by binding to Keap1, which is attached to the actin cytoskeleton<ref name="albena" />. Inducers disrupt the Keap1–Nrf2 complex, and Nrf2 migrates to the nucleus where it forms heterodimers with other transcription factors such as small Maf that bind to the ARE regions of phase 2 genes and accelerate their transcription. Several types of modifications of Keap1 by inducers are shown. | Nrf2 is anchored in the cytoplasm by binding to Keap1, which is attached to the actin cytoskeleton<ref name="albena" />. Inducers disrupt the Keap1–Nrf2 complex, and Nrf2 migrates to the nucleus where it forms heterodimers with other transcription factors such as small Maf that bind to the ARE regions of phase 2 genes and accelerate their transcription. Several types of modifications of Keap1 by inducers are shown. | ||
| Line 54: | Line 56: | ||
98, 3410–3415</ref> | 98, 3410–3415</ref> | ||
| - | According to Motohashi and Yamamoto,<ref name="super"/> Nrf2 is also an important regulator of oxidative-stress inducible genes, including heme oxygenase-1 and peroxiredoxin 1. In a 2002 study<ref> Cho, H-Y. et al. (2002) Role of NRF2 in protection against hyperoxic | + | According to Motohashi and Yamamoto,<ref name="super"/> Nrf2 is also an important regulator of oxidative-stress inducible genes, including heme oxygenase-1 and peroxiredoxin 1. In a 2002 study<ref> Cho, H-Y. et al. (2002) Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 26, 175–182</ref>, a single-nucleotide polymorphism was detected in the promoter region of the Nrf2 gene of the mouse strain C57BL/6J, which is sensitive to hyperoxic stress. Supporting this, Nrf2-null mutant mice were found to be highly susceptible to hyperoxic lung injury. The impaired defense mechanisms against oxidative stress that are observed in the nrf2-null mutant mice could have resulted from the accumulation of reactive oxygen species (ROS) in the absence of Nrf2. A combination of electron paramagnetic resonance (EPR) and spin-probe kinetic analysis confirmed that there is a substantial decrease in the ability of nrf2-null mutant liver and kidney to eliminate ROS. This impaired elimination of ROS was exacerbated in aging female animals. Consistent with this result, old female Nrf2-deficient mice with an ICR genetic background often developed severe lupus-like autoimmune nephritis. Because ROS have a prominent role in the pathogenesis of nephritis, the accumulation of ROS due to Nrf2 deficiency must have exacerbated the mild glomerular lesions that are inherent to the ICR strain of mice. |
| - | lung injury in mice. Am. J. Respir. Cell Mol. Biol. 26, 175–182</ref>, a single-nucleotide polymorphism was detected in the promoter region of the Nrf2 gene of the mouse strain C57BL/6J, which is sensitive to hyperoxic stress. Supporting this, Nrf2-null mutant mice were found to be highly susceptible to hyperoxic lung injury. The impaired defense mechanisms against oxidative stress that are observed in the nrf2-null mutant mice could have resulted from the accumulation of reactive oxygen species (ROS) in the absence of Nrf2. A combination of electron paramagnetic resonance (EPR) and spin-probe kinetic analysis confirmed that there is a substantial decrease in the ability of nrf2-null mutant liver and kidney to eliminate ROS. This impaired elimination of ROS was exacerbated in aging female animals. Consistent with this result, old female Nrf2-deficient mice with an ICR genetic background often developed severe lupus-like autoimmune nephritis. Because ROS have a prominent role in the pathogenesis of nephritis, the accumulation of ROS due to Nrf2 deficiency must have exacerbated the mild glomerular lesions that are inherent to the ICR strain of mice. | + | |
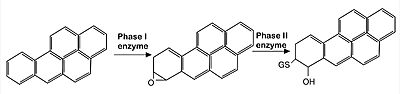
[[Image:Met.jpg|400px|center|]] | [[Image:Met.jpg|400px|center|]] | ||
Image: representation of endocytic metabolisation of reactive agents (in this case, Benoz[a]pyrine molecules). The xenobiotic agent is first oxidized by phase I enzymes belonging to the P450 mono-oxygenase system (such as CYP1A1 and CYP1A2 enzymes). Since the product of such reactions are often highly reactive, they are subsequently detoxified by the induction of enzymes related to Phase II reactions (such as glutathione S-transferase and UDP–glucuronosyl-transferase), that promote the conjugation of phase I products with hydrophilic moieties, such as glutathione and glucuronic acid, so that the final, non-reactive product can be then eliminated by excretion. Nrf2 is the main CNC transcription factor responsible for the induction of those Phase II enzymes. | Image: representation of endocytic metabolisation of reactive agents (in this case, Benoz[a]pyrine molecules). The xenobiotic agent is first oxidized by phase I enzymes belonging to the P450 mono-oxygenase system (such as CYP1A1 and CYP1A2 enzymes). Since the product of such reactions are often highly reactive, they are subsequently detoxified by the induction of enzymes related to Phase II reactions (such as glutathione S-transferase and UDP–glucuronosyl-transferase), that promote the conjugation of phase I products with hydrophilic moieties, such as glutathione and glucuronic acid, so that the final, non-reactive product can be then eliminated by excretion. Nrf2 is the main CNC transcription factor responsible for the induction of those Phase II enzymes. | ||
| - | == Structural and Genetic information of Nrf2 == | ||
| - | + | Thanks to the many binding sites of Nrf2, studies have shown that there are many alternative pathway from which Nrf2 degradation can occur, providing many mechanism for cellular control of Nrf2 activities <ref>Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034</ref>, subjected to Keap1 regulation or otherwise. The following diagram from Warren L. Wu and Thales Papagiannakopoulos <ref>https://www.annualreviews.org/doi/full/10.1146/annurev-cancerbio-030518-055627, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=94063579</ref> summarizes all known Nrf2 related metabolic processes. | |
| + | |||
| + | [[Image:Inputs_and_outputs_of_KEAP1_NRF2_pathway.jpg|600px|center|]] | ||
| + | == Structural and Genetic Information of Nrf2 == | ||
| - | + | NRF2 is 605 amino acids long and possesses <scene name='89/898348/Nrf2inteirafinal/1'>seven highly conserved domains</scene> called NRF2-ECH homology (Neh) domains <ref>Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034</ref>. Their names and function is as follows: | |
| - | <scene name='89/898348/ | + | <scene name='89/898348/Nrf2dom1/1'>Neh1 domain</scene> (orange): A CNC-bZIP domain that allows Nrf2 to heterodimerize with small <scene name='89/898348/Smaf/1'>Maf</scene> proteins. |
| - | + | <scene name='89/898348/Nrf2dom2/1'>Neh2 domain</scene> (dark blue): allows for <scene name='89/898348/Neh2ultima/1'>binding of NRF2 to Keap1, through to degrons called DLG and ETGE motifs. </scene>The Nrf2 peptide contains two short antiparallel β-strands connected by two overlapping type I β-turns stabilized by the aspartate and threonine residues. The β-turn region fits <scene name='89/898348/Flu2/1'>into a binding pocket</scene> on the top face of the Kelch domain and the glutamate residues form multiple hydrogen bonds with highly conserved residues in Keap1. | |
| - | Neh6 domain (yellow): may contain a degron that is involved in a redox-insensitive process of degradation of NRF2. This occurs even in stressed cells, which normally extend the half-life of NRF2 protein relative to unstressed conditions by suppressing other degradation pathways. | + | <scene name='89/898348/Nrf2dom3/1'>Neh3</scene>, <scene name='89/898348/Nrf2dom4/1'>Neh4</scene> and <scene name='89/898348/Nrf2dom5/1'>Neh5</scene> domain (red, blue and light blue): may play a role in NRF2 protein stability and may act as a transactivation domain, interacting with component of the transcriptional apparatus (Neh3) or to a protein called cAMP Response Element Binding Protein (Neh4 and Neh5), which possesses intrinsic histone acetyltransferase activity. |
| + | |||
| + | <scene name='89/898348/Nrf2dom6/2'>Neh6 domain</scene>(yellow): may contain a degron that is not recognized by Keap1, but it is instead involved in a redox-insensitive process of E3 ubiquitin ligase β-TrCP degradation of NRF2. This occurs even in stressed cells, which normally extend the half-life of NRF2 protein relative to unstressed conditions by suppressing other degradation pathways. | ||
| + | |||
| + | <scene name='89/898348/Nrf2dom7/1'>Nh7 domain </scene>(green): interacts with the DNA-binding domain of retinoic X receptor alfa. | ||
| + | [[Image:SequenciaNrf2.jpeg|500px|center|]] | ||
| - | Nh7 domain (green): | ||
The gene responsible for Nrf2 coding in humans is called HAGRID 283, and its sequence of bases is available in the [https://genomics.senescence.info/genes/entry.php?hgnc=NFE2L2 Human Ageing Genomic Resources] database, and it is as follows: | The gene responsible for Nrf2 coding in humans is called HAGRID 283, and its sequence of bases is available in the [https://genomics.senescence.info/genes/entry.php?hgnc=NFE2L2 Human Ageing Genomic Resources] database, and it is as follows: | ||
| - | [[Image:Nrf2gene.jpg| | + | [[Image:Nrf2gene.jpg|500px|center|]] |
| - | == | + | == Structural and Genetic Information of Keap1 == |
| + | Keap1 belongs to the metazoan superfamily of BTB-[https://en.wikipedia.org/wiki/Kelch_protein Kelch] proteins, a widespread group of proteins that contain multiple Kelch motifs. The Kelch domain generally occurs as a set of five to seven kelch tandem repeats that form a <scene name='89/898348/Betaprop/1'>β-propeller tertiary structure.</scene>. In <scene name='89/898348/Keap1mice/1'>mice</scene>, Keap1 has 624 amino acids, composing five different domains with the following names and caracteristics: | ||
| + | |||
| + | NTR: N-terminal region. | ||
| + | |||
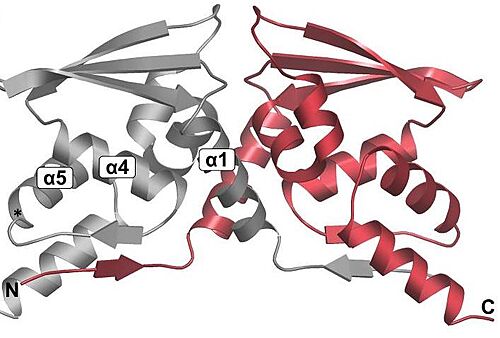
| + | <scene name='89/898348/Btb/1'>BTB</scene>: The BTB/POZ domain (bric-a-brac, tramtrack, broad complex/Poxvirus zinc finger) is an evolutionary conserved domain also found in actin-binding proteins, zinc finger transcription factors, and substrate specific adaptor proteins in Cullin3 (Cul3)-based E3 ubiquitin ligase complexes <ref name="res" />. In many cases, this is a protein-protein interaction domain that mediates dimerization, a process that is required for the binding with Nrf2. | ||
| + | [[Image:DimmerBTB.jpg|500px|center|]] | ||
| + | Overall fold of the Keap1 BTB crystallographic dimer as a cartoon representation. The N and C-termini, and key alpha-helical secondary structural elements are labelled for one BTB monomer.<ref>Cleasby A, Yon J, Day PJ, et al. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS One. 2014;9(6):e98896. Published 2014 Jun 4. doi:10.1371/journal.pone.0098896</ref>. In mice, Keap1 dimmers are considered zinc metalloprotein, since the coupling is help by stoichiometric amounts of zinc and cobalt. | ||
| + | |||
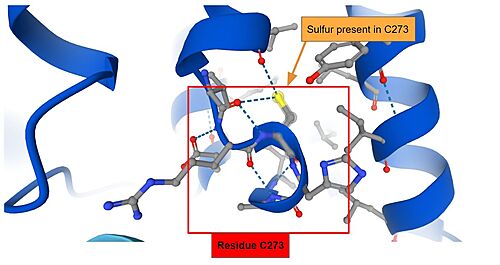
| + | IVR: It is in the IVR domain that most of the cysteine residues is found, which suggest that this domain is the sensor for reactive/oxidative agents, since cysteine residues are highly reactive. As mentioned previously, it has been established that in mice, the residues Cys273 and Cys288 — both present in the IVR domain — contain the sulfite ions that are capable of reacting to invasive agents<ref name="res" />. When the <scene name='89/898348/Keap1micecys/1'>sulfite terminals</scene> of C273 and C288 are perturbed by invasive substances, the Nrf2 that was once marked for ubiquitination is released and as such the cryoprotective reaction can occur. The sulfite present in Cys273 is represented bellow. | ||
| + | |||
| + | [[Image:ResidueS.jpg|500px|center|]] | ||
| + | |||
| + | DGR (Kelch):The <scene name='89/898348/Kelch/1'>Kelch domain</scene> is a monomer and is comprised of six Kelch repeats that form a symmetric, six-bladed beta-propeller structure. The structure reveals that the Kelch repeat motif is defined by highly conserved glycine, tyrosine, and tryptophan residues. There are eight cysteine residues, none of them engaged in disulfide bonds. Interestingly, the first blade of the propeller consists of three strands from the N terminus of the protein and one strand from the C terminus, thus bringing the carboxy terminal region of Keap1 into close proximity to the IVR. Even thought the Kelch domain is not responsible for sensing the introduction of oxidative substances (that is the role of Cys273 and Cys288 in the IVR domain), it is still a highly reactive site and it is through the Kelch domain that the Keap1 protein is able to <scene name='89/898348/Flu2/1'>bind itself to the Neh2 domain of Nrf2.</scene> | ||
| + | |||
| + | CTR: C-terminal region. This region is of interest because it ends with a -CTC tripeptide, which is conserved in the other three mammalian proteins. This motif is very similar to the -CXXC- sequence found in the active site | ||
| + | of protein disulfide isomerase. Woycechowsky and Raines <ref>Woycechowsky, K. J., and Raines, R. T. (2003) The CXC motif: a | ||
| + | functional mimic of protein disulfide isomerase. Biochemistry 42, | ||
| + | 5387-5394. </ref>have reported that the tripeptide CGCNH2 has a disulfide reduction potential similar to that of protein disulfide isomerase and even possesses disulfide isomerization activity. Whether the -CTC sequence in Keap1 has any disulfide reduction/isomerization activity has not yet been determined. | ||
== Structural highlights == | == Structural highlights == | ||
| - | Keap1 finalmente todos os dominios: | + | |
| + | Keap1 finalmente todos os dominios (humano): | ||
<scene name='89/898348/Finalmentekeap1/1'>Text To Be Displayed</scene> | <scene name='89/898348/Finalmentekeap1/1'>Text To Be Displayed</scene> | ||
| + | |||
| + | Keap1 finalmente todos os dominios (mice): | ||
| + | <scene name='89/898348/Keap1mice/1'>Text To Be Displayed</scene> | ||
| + | |||
| + | Keap1 (mice) com cys 273 e 288 destacados: | ||
| + | <scene name='89/898348/Keap1micecys/1'>Text To Be Displayed</scene> | ||
| + | |||
| + | BTB: | ||
| + | <scene name='89/898348/Btb/1'>Text To Be Displayed</scene> | ||
| + | |||
| + | Kelch: | ||
| + | <scene name='89/898348/Kelch/1'>Text To Be Displayed</scene> | ||
Nrf2 todos os dominios: | Nrf2 todos os dominios: | ||
| Line 93: | Line 131: | ||
Nrf2 dom6: | Nrf2 dom6: | ||
<scene name='89/898348/Nrf2dom6/2'>Text To Be Displayed</scene> | <scene name='89/898348/Nrf2dom6/2'>Text To Be Displayed</scene> | ||
| + | |||
| + | Nrf2 dom7: | ||
| + | <scene name='89/898348/Nrf2dom7/1'>Text To Be Displayed</scene> | ||
| + | |||
| + | Nrf2 dom5: | ||
| + | <scene name='89/898348/Nrf2dom5/1'>Text To Be Displayed</scene> | ||
| + | |||
| + | Nrf2 dom4: | ||
| + | <scene name='89/898348/Nrf2dom4/1'>Text To Be Displayed</scene> | ||
| + | |||
| + | Nrf2 dom2: | ||
| + | <scene name='89/898348/Nrf2dom2/1'>Text To Be Displayed</scene> | ||
| + | |||
| + | Maf: | ||
| + | <scene name='89/898348/Smaf/1'>Text To Be Displayed</scene> | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Keap1-Nrf2 Complex
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Lo, S.-C., Li, X., Henzl, M.T., Beamer, L.J. and Hannink, M. (2006), Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25: 3605-3617. https://doi.org/10.1038/sj.emboj.7601243
- ↑ Pitoniak, A., & Bohmann, D. (2015). Mechanisms and functions of Nrf2 signaling in Drosophila. Free radical biology & medicine, 88(Pt B), 302–313. https://doi.org/10.1016/j.freeradbiomed.2015.06.020
- ↑ Data available in https://www.ncbi.nlm.nih.gov/gene/9817
- ↑ Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;(71):157-76. doi: 10.1042/bss0710157. PMID: 15777020.
- ↑ Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001 Jun 2;477(1-2):7-21. doi: 10.1016/s0027-5107(01)00091-4. PMID: 11376682.
- ↑ Ceconi C, Boraso A, Cargnoni A, Ferrari R. Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys. 2003 Dec 15;420(2):217-21. doi: 10.1016/j.abb.2003.06.002. PMID: 14654060.
- ↑ Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003 Nov 28;278(48):48021-9. doi: 10.1074/jbc.M308439200. Epub 2003 Sep 10. PMID: 12968018.
- ↑ 8.0 8.1 Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11908-13. doi: 10.1073/pnas.172398899. Epub 2002 Aug 22. PMID: 12193649; PMCID: PMC129367.
- ↑ 9.0 9.1 9.2 Albena T. Dinkova-Kostova, W. David Holtzclaw, and Thomas W. Kensler. The Role of Keap1 in Cellular Protective Responses. American Chemical Society, August 2, 2005.
- ↑ Nobunao Wakabayashi, Albena T. Dinkova-Kostova, W. David Holtzclaw, Moon-Il Kang, Akira Kobayashi, Masayuki Yamamoto, Thomas W. Kensler, and Paul Talalay. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. PNAS February 17, 2004 vol. 101 no. 7.
- ↑ 11.0 11.1 11.2 Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004 Nov;10(11):549-57. doi: 10.1016/j.molmed.2004.09.003. PMID: 15519281.
- ↑ Sasaki, H. et al. (2002) Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J. Biol. Chem. 277, 44765–44771
- ↑ Chan, K. and Kan, Y.W. (1999) Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. U. S. A. 96, 12731–12736
- ↑ Enomoto, A. et al. (2001) High sensitivity of Nrf2 knockout mice to Review TRENDS in Molecular Medicine Vol.10 No.11 November 2004 555 www.sciencedirect.com acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 59, 169–177
- ↑ Goldring, C.E. et al. (2004) Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology 39, 1267–1276
- ↑ Ramos-Gomez, M. et al. (2001) Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 98, 3410–3415
- ↑ Cho, H-Y. et al. (2002) Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 26, 175–182
- ↑ Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034
- ↑ https://www.annualreviews.org/doi/full/10.1146/annurev-cancerbio-030518-055627, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=94063579
- ↑ Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034
- ↑ Cleasby A, Yon J, Day PJ, et al. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS One. 2014;9(6):e98896. Published 2014 Jun 4. doi:10.1371/journal.pone.0098896
- ↑ Woycechowsky, K. J., and Raines, R. T. (2003) The CXC motif: a functional mimic of protein disulfide isomerase. Biochemistry 42, 5387-5394.