User:Mathias Bortoletto Dunker/Sandbox 1
From Proteopedia
< User:Mathias Bortoletto Dunker(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
<StructureSection load='2Flu' size='340' side='right' caption='Crystal Structure of the Kelch-Neh2 Complex' scene='89/898348/Flu2/1'> | <StructureSection load='2Flu' size='340' side='right' caption='Crystal Structure of the Kelch-Neh2 Complex' scene='89/898348/Flu2/1'> | ||
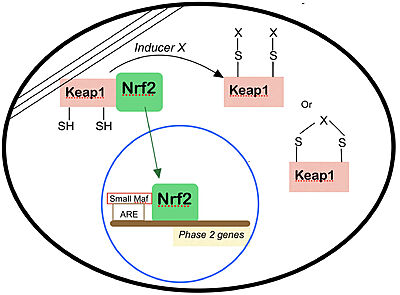
| - | Keap1 is a BTB-Kelch substrate adaptor protein anchored to the actin cytoskeleton that regulates steady-state levels of [https://en.wikipedia.org/wiki/NFE2L2 Nrf2],<ref name="main">Lo, S.-C., Li, X., Henzl, M.T., Beamer, L.J. and Hannink, M. (2006), Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25: 3605-3617. https://doi.org/10.1038/sj.emboj.7601243 </ref> one of the main transcription factor of cytoprotective genes found in mammals.<ref>Pitoniak, A., & Bohmann, D. (2015). Mechanisms and functions of Nrf2 signaling in Drosophila. Free radical biology & medicine, 88(Pt B), 302–313. https://doi.org/10.1016/j.freeradbiomed.2015.06.020</ref> Under homeostatic conditions, Keap1 is capable of marking Nfr2 for [https://en.wikipedia.org/wiki/Ubiquitin ubiquitin-dependent degradation], repressing the expression of genes associated with cytological defense against highly reactive molecules, such as electrophilic chemicals, heavy metals and oxidative agents. <ref name="main" /> As such, when the cell is subjected to stresses of those kinds, Nrf2 is no longer targeted for ubiquitin-dependent degradation, thus allowing for the expressions of the genes responsible for the cellular defense against such reactive substances. | + | Keap1 is a BTB-Kelch substrate adaptor protein anchored to the actin cytoskeleton that regulates steady-state levels of [https://en.wikipedia.org/wiki/NFE2L2 Nrf2],<ref name="main">Lo, S.-C., Li, X., Henzl, M.T., Beamer, L.J. and Hannink, M. (2006), Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25: 3605-3617. https://doi.org/10.1038/sj.emboj.7601243 </ref> one of the main transcription factor of cytoprotective genes found in mammals.<ref>Pitoniak, A., & Bohmann, D. (2015). Mechanisms and functions of Nrf2 signaling in Drosophila. Free radical biology & medicine, 88(Pt B), 302–313. https://doi.org/10.1016/j.freeradbiomed.2015.06.020</ref> Under homeostatic conditions, <scene name='89/898348/Flu2/1'>Keap1 is capable of marking Nfr2 </scene> for [https://en.wikipedia.org/wiki/Ubiquitin ubiquitin-dependent degradation], repressing the expression of genes associated with cytological defense against highly reactive molecules, such as electrophilic chemicals, heavy metals and oxidative agents. <ref name="main" /> As such, when the cell is subjected to stresses of those kinds, Nrf2 is no longer targeted for ubiquitin-dependent degradation, thus allowing for the expressions of the genes responsible for the cellular defense against such reactive substances. |
{| class="wikitable" | {| class="wikitable" | ||
| Line 68: | Line 68: | ||
NRF2 is 605 amino acids long and possesses <scene name='89/898348/Nrf2inteirafinal/1'>seven highly conserved domains</scene> called NRF2-ECH homology (Neh) domains <ref>Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034</ref>. Their names and function is as follows: | NRF2 is 605 amino acids long and possesses <scene name='89/898348/Nrf2inteirafinal/1'>seven highly conserved domains</scene> called NRF2-ECH homology (Neh) domains <ref>Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034</ref>. Their names and function is as follows: | ||
| - | <scene name='89/898348/Nrf2dom1/1'>Neh1 domain</scene> (orange): A CNC-bZIP domain that allows Nrf2 to heterodimerize with small Maf proteins. | + | <scene name='89/898348/Nrf2dom1/1'>Neh1 domain</scene> (orange): A CNC-bZIP domain that allows Nrf2 to heterodimerize with small <scene name='89/898348/Smaf/1'>Maf</scene> proteins. |
| - | <scene name='89/898348/Nrf2dom2/1'>Neh2 domain</scene> (dark blue): allows for <scene name='89/898348/Neh2ultima/1'>binding of NRF2 to Keap1, through | + | <scene name='89/898348/Nrf2dom2/1'>Neh2 domain</scene> (dark blue): allows for <scene name='89/898348/Neh2ultima/1'>binding of NRF2 to Keap1, through to degrons called DLG and ETGE motifs. </scene>The Nrf2 peptide contains two short antiparallel β-strands connected by two overlapping type I β-turns stabilized by the aspartate and threonine residues. The β-turn region fits <scene name='89/898348/Flu2/1'>into a binding pocket</scene> on the top face of the Kelch domain and the glutamate residues form multiple hydrogen bonds with highly conserved residues in Keap1. |
<scene name='89/898348/Nrf2dom3/1'>Neh3</scene>, <scene name='89/898348/Nrf2dom4/1'>Neh4</scene> and <scene name='89/898348/Nrf2dom5/1'>Neh5</scene> domain (red, blue and light blue): may play a role in NRF2 protein stability and may act as a transactivation domain, interacting with component of the transcriptional apparatus (Neh3) or to a protein called cAMP Response Element Binding Protein (Neh4 and Neh5), which possesses intrinsic histone acetyltransferase activity. | <scene name='89/898348/Nrf2dom3/1'>Neh3</scene>, <scene name='89/898348/Nrf2dom4/1'>Neh4</scene> and <scene name='89/898348/Nrf2dom5/1'>Neh5</scene> domain (red, blue and light blue): may play a role in NRF2 protein stability and may act as a transactivation domain, interacting with component of the transcriptional apparatus (Neh3) or to a protein called cAMP Response Element Binding Protein (Neh4 and Neh5), which possesses intrinsic histone acetyltransferase activity. | ||
| Line 89: | Line 89: | ||
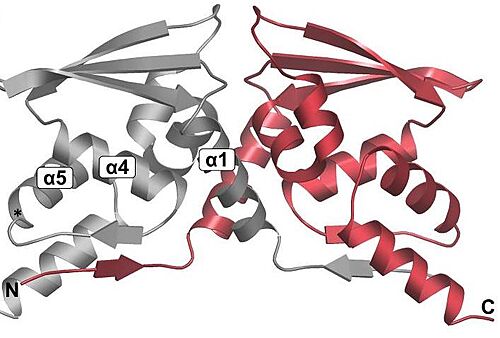
<scene name='89/898348/Btb/1'>BTB</scene>: The BTB/POZ domain (bric-a-brac, tramtrack, broad complex/Poxvirus zinc finger) is an evolutionary conserved domain also found in actin-binding proteins, zinc finger transcription factors, and substrate specific adaptor proteins in Cullin3 (Cul3)-based E3 ubiquitin ligase complexes <ref name="res" />. In many cases, this is a protein-protein interaction domain that mediates dimerization, a process that is required for the binding with Nrf2. | <scene name='89/898348/Btb/1'>BTB</scene>: The BTB/POZ domain (bric-a-brac, tramtrack, broad complex/Poxvirus zinc finger) is an evolutionary conserved domain also found in actin-binding proteins, zinc finger transcription factors, and substrate specific adaptor proteins in Cullin3 (Cul3)-based E3 ubiquitin ligase complexes <ref name="res" />. In many cases, this is a protein-protein interaction domain that mediates dimerization, a process that is required for the binding with Nrf2. | ||
[[Image:DimmerBTB.jpg|500px|center|]] | [[Image:DimmerBTB.jpg|500px|center|]] | ||
| - | Overall fold of the Keap1 BTB crystallographic dimer as a cartoon representation. The N and C-termini, and key alpha-helical secondary structural elements are labelled for one BTB monomer.<ref>Cleasby A, Yon J, Day PJ, et al. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS One. 2014;9(6):e98896. Published 2014 Jun 4. doi:10.1371/journal.pone.0098896</ref>. | + | Overall fold of the Keap1 BTB crystallographic dimer as a cartoon representation. The N and C-termini, and key alpha-helical secondary structural elements are labelled for one BTB monomer.<ref>Cleasby A, Yon J, Day PJ, et al. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS One. 2014;9(6):e98896. Published 2014 Jun 4. doi:10.1371/journal.pone.0098896</ref>. In mice, Keap1 dimmers are considered zinc metalloprotein, since the coupling is help by stoichiometric amounts of zinc and cobalt. |
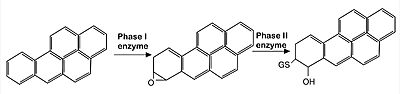
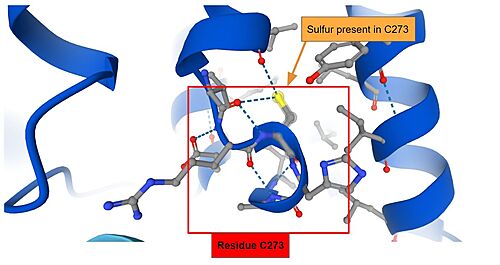
IVR: It is in the IVR domain that most of the cysteine residues is found, which suggest that this domain is the sensor for reactive/oxidative agents, since cysteine residues are highly reactive. As mentioned previously, it has been established that in mice, the residues Cys273 and Cys288 — both present in the IVR domain — contain the sulfite ions that are capable of reacting to invasive agents<ref name="res" />. When the <scene name='89/898348/Keap1micecys/1'>sulfite terminals</scene> of C273 and C288 are perturbed by invasive substances, the Nrf2 that was once marked for ubiquitination is released and as such the cryoprotective reaction can occur. The sulfite present in Cys273 is represented bellow. | IVR: It is in the IVR domain that most of the cysteine residues is found, which suggest that this domain is the sensor for reactive/oxidative agents, since cysteine residues are highly reactive. As mentioned previously, it has been established that in mice, the residues Cys273 and Cys288 — both present in the IVR domain — contain the sulfite ions that are capable of reacting to invasive agents<ref name="res" />. When the <scene name='89/898348/Keap1micecys/1'>sulfite terminals</scene> of C273 and C288 are perturbed by invasive substances, the Nrf2 that was once marked for ubiquitination is released and as such the cryoprotective reaction can occur. The sulfite present in Cys273 is represented bellow. | ||
| Line 95: | Line 95: | ||
[[Image:ResidueS.jpg|500px|center|]] | [[Image:ResidueS.jpg|500px|center|]] | ||
| - | DGR (Kelch):The <scene name='89/898348/Kelch/1'>Kelch domain</scene> is a monomer and is comprised of six Kelch repeats that form a symmetric, six-bladed beta-propeller structure. The structure reveals that the Kelch repeat motif is defined by highly conserved glycine, tyrosine, and tryptophan residues. There are eight cysteine residues, none of them engaged in disulfide bonds. Interestingly, the first blade of the propeller consists of three strands from the N terminus of the protein and one strand from the C terminus, thus bringing the carboxy terminal region of Keap1 into close proximity to the IVR. Even thought the Kelch domain is not responsible for sensing the introduction of oxidative substances (that is the role of Cys273 and Cys288 in the IVR domain), it is still a highly reactive site and it is through the Kelch domain that the Keap1 protein is able to <scene name='89/898348/Flu2/1'>bind itself to the Neh2 domain of Nrf2.</scene> | + | DGR (Kelch):The <scene name='89/898348/Kelch/1'>Kelch domain</scene> is a monomer and is comprised of six Kelch repeats that form a symmetric, six-bladed beta-propeller structure. The structure reveals that the Kelch repeat motif is defined by highly conserved glycine, tyrosine, and tryptophan residues. There are eight cysteine residues, none of them engaged in disulfide bonds. Interestingly, the first blade of the propeller consists of three strands from the N terminus of the protein and one strand from the C terminus, thus bringing the carboxy terminal region of Keap1 into close proximity to the IVR. Even thought the Kelch domain is not responsible for sensing the introduction of oxidative substances (that is the role of Cys273 and Cys288 in the IVR domain), it is still a highly reactive site and it is through the Kelch domain that the Keap1 protein is able to <scene name='89/898348/Flu2/1'>bind itself to the Neh2 domain of Nrf2.</scene> |
| - | CTR: C-terminal region. This region is of interest because it ends with a -CTC tripeptide, which is conserved in | + | CTR: C-terminal region. This region is of interest because it ends with a -CTC tripeptide, which is conserved in the other three mammalian proteins. This motif is very similar to the -CXXC- sequence found in the active site |
| - | the other three mammalian proteins. This motif is very similar to the -CXXC- sequence found in the active site | + | |
of protein disulfide isomerase. Woycechowsky and Raines <ref>Woycechowsky, K. J., and Raines, R. T. (2003) The CXC motif: a | of protein disulfide isomerase. Woycechowsky and Raines <ref>Woycechowsky, K. J., and Raines, R. T. (2003) The CXC motif: a | ||
functional mimic of protein disulfide isomerase. Biochemistry 42, | functional mimic of protein disulfide isomerase. Biochemistry 42, | ||
| Line 144: | Line 143: | ||
Nrf2 dom2: | Nrf2 dom2: | ||
<scene name='89/898348/Nrf2dom2/1'>Text To Be Displayed</scene> | <scene name='89/898348/Nrf2dom2/1'>Text To Be Displayed</scene> | ||
| + | |||
| + | Maf: | ||
| + | <scene name='89/898348/Smaf/1'>Text To Be Displayed</scene> | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Keap1-Nrf2 Complex
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Lo, S.-C., Li, X., Henzl, M.T., Beamer, L.J. and Hannink, M. (2006), Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25: 3605-3617. https://doi.org/10.1038/sj.emboj.7601243
- ↑ Pitoniak, A., & Bohmann, D. (2015). Mechanisms and functions of Nrf2 signaling in Drosophila. Free radical biology & medicine, 88(Pt B), 302–313. https://doi.org/10.1016/j.freeradbiomed.2015.06.020
- ↑ Data available in https://www.ncbi.nlm.nih.gov/gene/9817
- ↑ Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;(71):157-76. doi: 10.1042/bss0710157. PMID: 15777020.
- ↑ Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001 Jun 2;477(1-2):7-21. doi: 10.1016/s0027-5107(01)00091-4. PMID: 11376682.
- ↑ Ceconi C, Boraso A, Cargnoni A, Ferrari R. Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys. 2003 Dec 15;420(2):217-21. doi: 10.1016/j.abb.2003.06.002. PMID: 14654060.
- ↑ Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003 Nov 28;278(48):48021-9. doi: 10.1074/jbc.M308439200. Epub 2003 Sep 10. PMID: 12968018.
- ↑ 8.0 8.1 Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11908-13. doi: 10.1073/pnas.172398899. Epub 2002 Aug 22. PMID: 12193649; PMCID: PMC129367.
- ↑ 9.0 9.1 9.2 Albena T. Dinkova-Kostova, W. David Holtzclaw, and Thomas W. Kensler. The Role of Keap1 in Cellular Protective Responses. American Chemical Society, August 2, 2005.
- ↑ Nobunao Wakabayashi, Albena T. Dinkova-Kostova, W. David Holtzclaw, Moon-Il Kang, Akira Kobayashi, Masayuki Yamamoto, Thomas W. Kensler, and Paul Talalay. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. PNAS February 17, 2004 vol. 101 no. 7.
- ↑ 11.0 11.1 11.2 Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004 Nov;10(11):549-57. doi: 10.1016/j.molmed.2004.09.003. PMID: 15519281.
- ↑ Sasaki, H. et al. (2002) Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J. Biol. Chem. 277, 44765–44771
- ↑ Chan, K. and Kan, Y.W. (1999) Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. U. S. A. 96, 12731–12736
- ↑ Enomoto, A. et al. (2001) High sensitivity of Nrf2 knockout mice to Review TRENDS in Molecular Medicine Vol.10 No.11 November 2004 555 www.sciencedirect.com acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 59, 169–177
- ↑ Goldring, C.E. et al. (2004) Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology 39, 1267–1276
- ↑ Ramos-Gomez, M. et al. (2001) Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 98, 3410–3415
- ↑ Cho, H-Y. et al. (2002) Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 26, 175–182
- ↑ Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034
- ↑ https://www.annualreviews.org/doi/full/10.1146/annurev-cancerbio-030518-055627, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=94063579
- ↑ Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034
- ↑ Cleasby A, Yon J, Day PJ, et al. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS One. 2014;9(6):e98896. Published 2014 Jun 4. doi:10.1371/journal.pone.0098896
- ↑ Woycechowsky, K. J., and Raines, R. T. (2003) The CXC motif: a functional mimic of protein disulfide isomerase. Biochemistry 42, 5387-5394.