Methionine synthase
From Proteopedia
(→Structural highlights) |
|||
| (92 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | __TOC__ | |
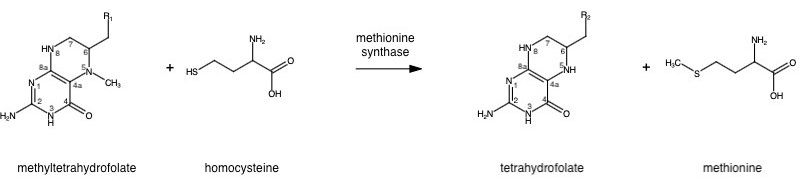
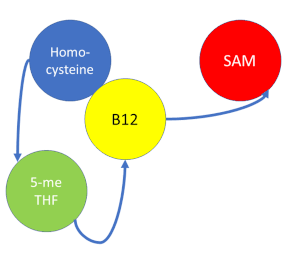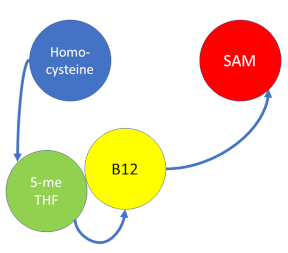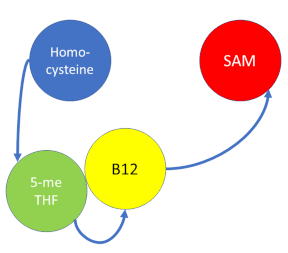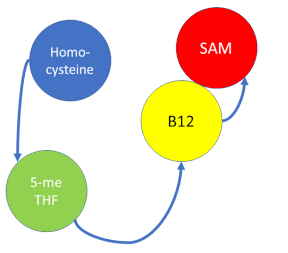
| + | '''Methionine synthase''' (MS; EC: 2.1.1.13) or '''5-methyltetrahydrofolate S-homocysteine methyltransferase''' is the enzyme in [[one-carbon metabolism]] linking the folate cycle to the methionine cycle. MS catalyzes the transfer of a methyl group from 5-methyltetrahydrofolate (5- me THF) to homocysteine, resulting in the formation of methionine and tetrahydrofolate (THF). Methionine is an essential amino acid required by our bodies for healthy cell and tissue growth. It is essential as it is not naturally derived in our bodies. As it is used as a methyl donor in the form of S-adenosylmethionine, the resulting homocysteine is recyled to form methionine again. | ||
| - | + | [[Image:Overall.jpg]] | |
| + | == Function == | ||
| - | = | + | MS is a B12-dependent enzyme responsible for regenerating methionine from homocysteine. MS uses vitamin B12 Cobalamin as a cofactor. The change from homocysteine to methionine is an SN2 reaction where the methyl group on N-5 from 5-me THF is donated to Cob(I)alamin forming methylcobalamin (or Me-Cob(III)alamin). This is a complex reaction as THF, a product, is a poor leaving group and requires the "super nucleophile", Cob(I)alamin, to carry out the reaction<ref>DOI:10.1146/annurev.biochem.72.121801.161828</ref><ref name="Kung et al">DOI: 10.1038/nature10916</ref> as a methyl carrier. |
| - | [[ | + | 5-me THF is a product of [[methylenetetrahydrofolate reductase]] ([[MTHFR]]) from the folate cycle. |
| - | + | == Oxidation States of Cobalamin == | |
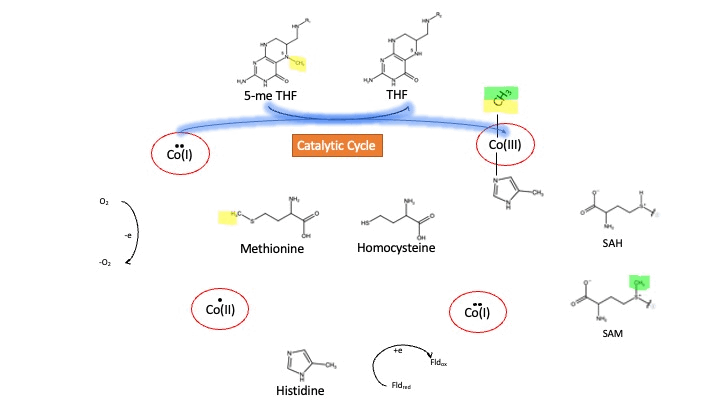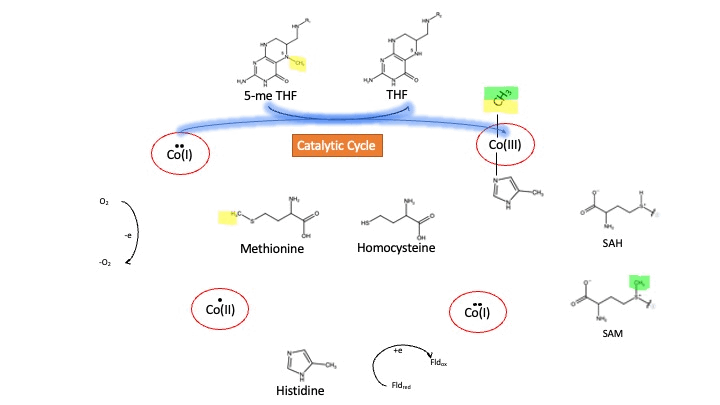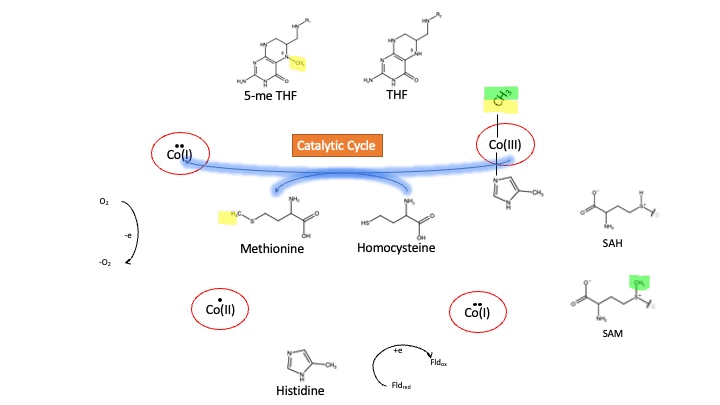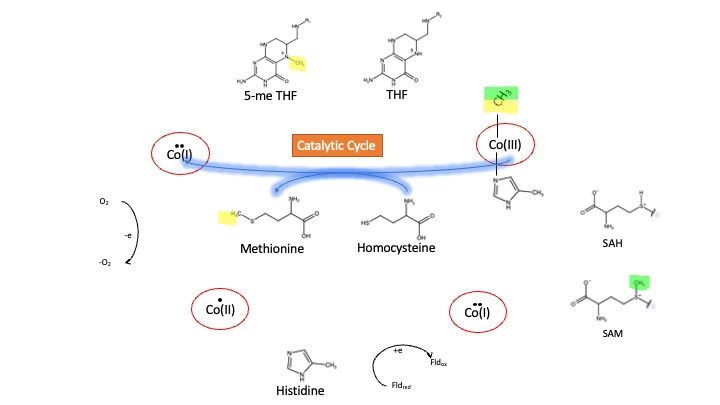
| - | + | [[Image: MS_cycle.gif]] | |
| - | + | Cobalamin exists in three different oxidation states during the MS cycle. | |
| - | Cob(I)alamin is required | + | |
| + | '''Cob(I)alamin''': Cobalt in the +1 oxidation state is nicknamed the "super nucleophile" as its high energy is required to carry out the complex SN2 reaction of breaking the bond between THF and the methyl group, in the catalytic cycle. | ||
| + | |||
| + | '''Co(III)alamin''': Cobalt in +3 oxidation state occurs when His 759 displaces the dimethylbenzimidazole (DMB) ligand to allow for the methyl to be accepted by Cob(I)alamin, forming Me-Cob(III)alamin. | ||
| + | |||
| + | '''Cob(II)alamin''': Cob(I)alamin is highly reactive towards oxygen so occasionally under aerobic conditions, Cob(I)alamin will undergo oxidation leading to an inactive Cob(II)alamin enzyme in the +2 oxidation state. This is regulated by reductive methylation by using Flavodoxin as an electron donor to reactivate Cob(I)alamin, and subsequently regenerates Me-Cob(III)alamin with a methyl being donated from SAM<ref>DOI:10.1073/pnas.1133218100</ref>. | ||
| - | + | == Relevance == | |
| - | + | MS is an important enzyme responsible for generating methionine, required by our bodies for healthy cell and tissue growth, and protein synthesis. Any MS and/or B12 deficiencies can result in diseases such as abnormal birth defects or anemia<ref name="Kung et al"/>. | |
| - | + | ||
| + | == Structural highlights == | ||
| - | == | + | ==Methionine synthase 3D structures== |
| - | + | [[Methionine synthase 3D structures]] | |
| + | <StructureSection load='' size='400' side='right' scene='90/907471/Superposition_1/3'> | ||
| - | == | + | === Domain organization === |
| - | + | ||
| - | + | ||
| - | = | + | Methionine synthase contains four domains, each with a unique function that bind to Cob(I)alamin as the methyl carrier. In the N-terminal, 5-me THF donates a methyl in the catalytic cycle to Cob(I)alamin, which then donates it to homocysteine to form methionine. However, every 2,000 cycles or so, Cob(I)alamin becomes oxidized (as shown below in the darker yellow color) and now requires reduction and remethylation triggering the reactivation cycle. In the C-terminal, S-adenosylmethionine or SAM donates methyl with Flavodoxin as the electron donor<ref name="Bandarian et al">DOI: 10.1038/nsb738</ref>. |
| - | + | [[Image:Methionine synthase domains.gif]] | |
| - | + | The full structure of MS has yet to be determined. | |
| - | + | Shown here is the <scene name='90/907471/Superposition/8'>theoretical prediction</scene> of the structure by the [[alphafold]] algorithm, with experimental structures of the N-terminal 2 domains as well as of the C-terminal 2 domins superposed. | |
| - | + | <jmol> | |
| + | <jmolRadioGroup> | ||
| + | <item> | ||
| + | <script>hide 1.1</script> | ||
| + | <text>experimental fragments</text> | ||
| + | <checked>false</checked> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>display 1.1</script> | ||
| + | <text>theoretical</text> | ||
| + | <checked>false</checked> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>display all</script> | ||
| + | <text>both</text> | ||
| + | <checked>true</checked> | ||
| + | </item> | ||
| + | </jmolRadioGroup> | ||
| + | </jmol> | ||
| - | + | As the graph below shows, the prediction has high confidence in the internal structure of individual domains but not the relative orientation. | |
| - | + | [[Image:Position error alphafold P13009.jpg|400px]] | |
| - | + | ||
| - | + | During each cycle, the domains must be positioned close enough to Cobalamin in order for methyl transfers to be successful. Conformations of MS allows substrates to be presented to Cobalamin for reactions to occur. | |
| - | + | ||
| + | === Cobalamin binding === | ||
| + | |||
| + | [[Image:cob_1_alamin.jpeg|300px]] | ||
| + | |||
| + | The Cobalamin binding domain has a special characteristic in that, it is most naturally found in a protective conformation to prevent unwanted chemistry from occurring (PDB: 1BMT). This is referred to as a 'capping' mechanism. | ||
| + | |||
| + | DMB, in the lower ligand of the <scene name='90/907471/Bindingdomain2/2'>B12 binding domain</scene> is displaced from the Cobalt by a Histidine residue to be 'uncapped' to form Me-Cob(III)alamin<ref>DOI:10.1146/annurev.biochem.72.121801.161828</ref>. | ||
| + | |||
| + | === Cap domain === | ||
| + | |||
| + | When B12 is not engaged with one of the other three substrate binding domains, it is protected by a <scene name='90/907471/Cap/1'>cap</scene>. | ||
| + | |||
| + | In the Cob(I)alamin binding domain, the <scene name='90/907471/Cap/5'>imidazole side chain containing His 759</scene> comes in and out of the B12 domain as the 5th ligand. His 759 bonds to Asp 757 and Ser 810 via hydrogen bonds to create a ligand triad that increases the efficiency of the methyl transfer during the catalytic cycle (not shown). With His on, the cap is on Cobalamin. When interacting with the activation domain, there is no room for the cap, and the Cobalamin moves a bit out of the reach of the Histidine (in the crystal structure, they used the His759Gly mutation to favor the His-off conformation).<ref name="Bandarian et al"/>. | ||
| + | |||
| + | <jmol> | ||
| + | <jmolRadioGroup> | ||
| + | <item> | ||
| + | <script>anim off; delay 1.0; model 2</script> | ||
| + | <text>capped</text> | ||
| + | <checked>true</checked> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>anim off; delay 1.0; model 1</script> | ||
| + | <text>with activation domain</text> | ||
| + | <checked>false</checked> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>anim off; delay 1.0; model 0;</script> | ||
| + | <text>both</text> | ||
| + | <checked>false</checked> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>anim fps 1; anim mode loop; anim on;</script> | ||
| + | <text>animate</text> | ||
| + | <checked>false</checked> | ||
| + | </item> | ||
| + | </jmolRadioGroup> | ||
| + | </jmol> | ||
| + | |||
| + | === Cobalamin activation === | ||
| + | |||
| + | Every 2000 or so cycles, cobalamin needs to be <scene name='90/907471/B12_activation_w_sah/2'>reactivated</scene> through methylation by S-adenosyl methionine (SAM). To determine the structure of the reactivation conformation, the mutant H759G was used. This mutation maximises the fraction of enzyme with the B12 domain in the cap-off conformation bound to the activation domain. The approach of the B12 domain and the activation domain has to be carefully regulated because methylating homocysteine with methyl groups from S-adenosyl methionine results in a futile cycle. Thus, this step should be reserved to rescue B12 out of the +2 cobalt oxidation state, and then methylation of homocysteine using a methyl group from 5-me THF resumes. | ||
</StructureSection> | </StructureSection> | ||
| + | |||
| + | == Acknowledgements == | ||
| + | |||
| + | Many thanks to Dr. Theis, Anna, Mike, and Shaylie for their enthusiasm and assistance on creation of 3D structural images of the B12 domain for MS. | ||
| + | |||
| + | Also thanks to Dr. Drennan for taking the time to review the page and providing helpful suggestions for improvement. | ||
== References == | == References == | ||
| - | <ref>DOI: 10.1128/JB.00208-06</ref> | ||
<references/> | <references/> | ||
| + | [[Category:One-carbon metabolism]] | ||
Current revision
Contents |
Methionine synthase (MS; EC: 2.1.1.13) or 5-methyltetrahydrofolate S-homocysteine methyltransferase is the enzyme in one-carbon metabolism linking the folate cycle to the methionine cycle. MS catalyzes the transfer of a methyl group from 5-methyltetrahydrofolate (5- me THF) to homocysteine, resulting in the formation of methionine and tetrahydrofolate (THF). Methionine is an essential amino acid required by our bodies for healthy cell and tissue growth. It is essential as it is not naturally derived in our bodies. As it is used as a methyl donor in the form of S-adenosylmethionine, the resulting homocysteine is recyled to form methionine again.
Function
MS is a B12-dependent enzyme responsible for regenerating methionine from homocysteine. MS uses vitamin B12 Cobalamin as a cofactor. The change from homocysteine to methionine is an SN2 reaction where the methyl group on N-5 from 5-me THF is donated to Cob(I)alamin forming methylcobalamin (or Me-Cob(III)alamin). This is a complex reaction as THF, a product, is a poor leaving group and requires the "super nucleophile", Cob(I)alamin, to carry out the reaction[1][2] as a methyl carrier.
5-me THF is a product of methylenetetrahydrofolate reductase (MTHFR) from the folate cycle.
Oxidation States of Cobalamin
Cobalamin exists in three different oxidation states during the MS cycle.
Cob(I)alamin: Cobalt in the +1 oxidation state is nicknamed the "super nucleophile" as its high energy is required to carry out the complex SN2 reaction of breaking the bond between THF and the methyl group, in the catalytic cycle.
Co(III)alamin: Cobalt in +3 oxidation state occurs when His 759 displaces the dimethylbenzimidazole (DMB) ligand to allow for the methyl to be accepted by Cob(I)alamin, forming Me-Cob(III)alamin.
Cob(II)alamin: Cob(I)alamin is highly reactive towards oxygen so occasionally under aerobic conditions, Cob(I)alamin will undergo oxidation leading to an inactive Cob(II)alamin enzyme in the +2 oxidation state. This is regulated by reductive methylation by using Flavodoxin as an electron donor to reactivate Cob(I)alamin, and subsequently regenerates Me-Cob(III)alamin with a methyl being donated from SAM[3].
Relevance
MS is an important enzyme responsible for generating methionine, required by our bodies for healthy cell and tissue growth, and protein synthesis. Any MS and/or B12 deficiencies can result in diseases such as abnormal birth defects or anemia[2].
Structural highlights
Methionine synthase 3D structures
Methionine synthase 3D structures
| |||||||||||
Acknowledgements
Many thanks to Dr. Theis, Anna, Mike, and Shaylie for their enthusiasm and assistance on creation of 3D structural images of the B12 domain for MS.
Also thanks to Dr. Drennan for taking the time to review the page and providing helpful suggestions for improvement.
References
- ↑ Banerjee R, Ragsdale SW. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72:209-47. doi: 10.1146/annurev.biochem.72.121801.161828. PMID:14527323 doi:http://dx.doi.org/10.1146/annurev.biochem.72.121801.161828
- ↑ 2.0 2.1 Kung Y, Ando N, Doukov TI, Blasiak LC, Bender G, Seravalli J, Ragsdale SW, Drennan CL. Visualizing molecular juggling within a B(12)-dependent methyltransferase complex. Nature. 2012 Mar 14. doi: 10.1038/nature10916. PMID:22419154 doi:10.1038/nature10916
- ↑ Bandarian V, Ludwig ML, Matthews RG. Factors modulating conformational equilibria in large modular proteins: a case study with cobalamin-dependent methionine synthase. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8156-63. doi:, 10.1073/pnas.1133218100. Epub 2003 Jun 27. PMID:12832615 doi:http://dx.doi.org/10.1073/pnas.1133218100
- ↑ 4.0 4.1 Bandarian V, Pattridge KA, Lennon BW, Huddler DP, Matthews RG, Ludwig ML. Domain alternation switches B(12)-dependent methionine synthase to the activation conformation. Nat Struct Biol. 2002 Jan;9(1):53-6. PMID:11731805 doi:10.1038/nsb738
- ↑ Banerjee R, Ragsdale SW. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72:209-47. doi: 10.1146/annurev.biochem.72.121801.161828. PMID:14527323 doi:http://dx.doi.org/10.1146/annurev.biochem.72.121801.161828
Proteopedia Page Contributors and Editors (what is this?)
Kia Yang, Karsten Theis, Michal Harel, Anna Postnikova, Michael O'Shaughnessy