Dihydrofolate reductase
From Proteopedia
| (2 intermediate revisions not shown.) | |||
| Line 89: | Line 89: | ||
=== Inhibitors === | === Inhibitors === | ||
| - | The human enzyme is sufficiently different from the bacterial enzymes to make specific inhibitors<ref>DOI:10.1038/s41429-019-0240-6</ref>. Inhibitors of the human enzyme are used in cancer treatment while inhibitors of the bacterials enzymes<ref>PMID:27352799</ref> are used as broad-spectrum antibiotics or more specifically to treat a specific infectious disease. An example of in inhibitor of bacterial DHFR is Trimethoprim (TMP). | + | The human enzyme is sufficiently different from the bacterial enzymes to make specific inhibitors<ref>DOI:10.1038/s41429-019-0240-6</ref>. Inhibitors of the human enzyme are used in cancer treatment while inhibitors of the bacterials enzymes<ref>PMID:27352799</ref> are used as broad-spectrum antibiotics or more specifically to treat a specific infectious disease. An example of in inhibitor of bacterial DHFR is [[Trimethoprim]] (TMP). |
Antifolate inhibitors like <scene name='82/82636/Mtx_inhibitor/2'>Methotrexate</scene> (MTX), which is very similar to <scene name='82/82636/Substrate_product/1'>dihydrofolate and tetrahydrofolate</scene>, are used in cancer therapy, see also [[Methotrexate]]. | Antifolate inhibitors like <scene name='82/82636/Mtx_inhibitor/2'>Methotrexate</scene> (MTX), which is very similar to <scene name='82/82636/Substrate_product/1'>dihydrofolate and tetrahydrofolate</scene>, are used in cancer therapy, see also [[Methotrexate]]. | ||
=== Resistance === | === Resistance === | ||
| - | Antibiotics that inhibit bacterial DHFR are widely used in medicine and agriculture, and some bacterial strains have acquired resistance<ref>DOI:10.3109/14756366.2013.787422</ref>. For example, some bacteria carry (either on their genome or on a plasmid) the trimethoprim-resistant ''dfrB'' gene coding for an enzyme DfrB that has a fold distinct from DHFR (coded by the ''folA'' gene)<ref>PMID:7583655</ref>. Like DHFR, Dfrb has dihydrofolate reductase activity, but unlike DHFR, it is not inhibited by trimethoprim. Thus, resistant bacteria can grow even in the presence of | + | Antibiotics that inhibit bacterial DHFR are widely used in medicine and agriculture, and some bacterial strains have acquired resistance<ref>DOI:10.3109/14756366.2013.787422</ref>. For example, some bacteria carry (either on their genome or on a plasmid) the trimethoprim-resistant ''dfrB'' gene coding for an enzyme DfrB that has a fold distinct from DHFR (coded by the ''folA'' gene)<ref>PMID:7583655</ref>. Like DHFR, Dfrb has dihydrofolate reductase activity, but unlike DHFR, it is not inhibited by trimethoprim. Thus, resistant bacteria can grow even in the presence of [[Trimethoprim]], relying on the Dfrb enzyme while the DHFR enzyme is inhibited. The <scene name='82/82636/Dfrb/1'>Dfrb structure</scene> is unique in that the active site has fourfold symmetry, with DHF and NADPH bound by the same amino acid residues from different subunits. In the available crystal structure, two folate molecules are bound (use checkbox to show). The enzyme is non-specific and can bind to unproductive combinations of ligands (two DHF molecules or two NADPH molecules) or to the productive combination of one DHF and one NADPH molecule each. |
<jmol> | <jmol> | ||
| Line 120: | Line 120: | ||
== Acknowledgements == | == Acknowledgements == | ||
| - | This page was revised as part of the Spring 2022 Biochemistry II course at [https://www.westfield.ma.edu/ WSU]. Thanks go to Kia, | + | This page was revised as part of the Spring 2022 Biochemistry II course at [https://www.westfield.ma.edu/ WSU]. Thanks go to [[User:Kia Yang|Kia]], [[User:Anna Postnikova|Anna]], [[User:Shaylie Albright|Shaylie]] and [[User:Michael O'Shaughnessy|Michael]] for helpful suggestions to improve the page. |
== References == | == References == | ||
Current revision
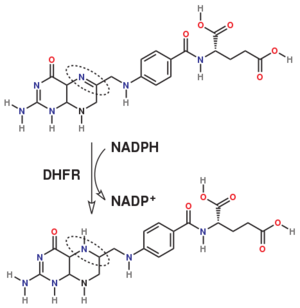
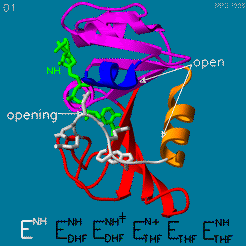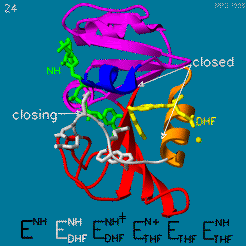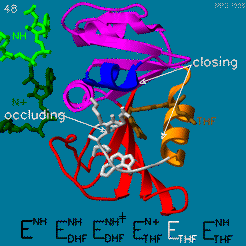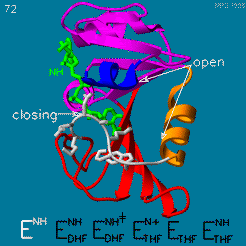
The enzyme dihydrofolate reductase (DHFR) occurs in all organisms and has been particularly well-studied in the bacterium Escherichia coli and in humans[1][2][3]. It catalyzes the reduction of dihydrofolate to tetrahydrofolate, with NADPH acting as hydride donor. The human enzyme is a target for developing inhibitors used in anti-cancer chemotherapies[4], while the bacterial enzymes are targets for developing inhibitors as antibiotics. DHFR is a model enzyme for studying the kinetics, mechanism, and inhibition of enzymatic reactions and the underlying structure and conformational dynamics.
Contents |
DHFR occurs in all organisms and most cells
DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli (ecDHFR) and humans (hDHFR) have similar folds, while the plasmid-encoded enzyme has an unrelated fold. In humans, DHFR is expressed in most tissues[1], and there are two genes, DHFR and DHFR2/DHFRL1, the latter targeted to mitochondria[5]. Mice and rats lack the second gene but also show DHFR activity in mitochondria[6].
Reactions catalyzed
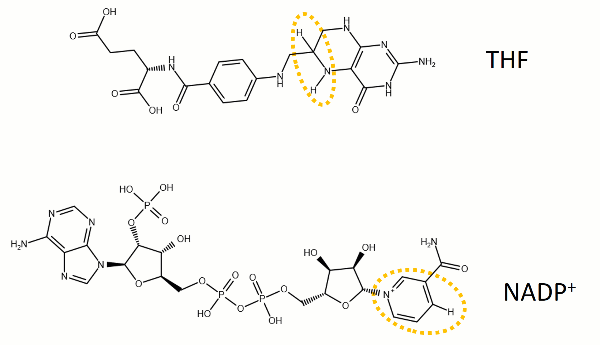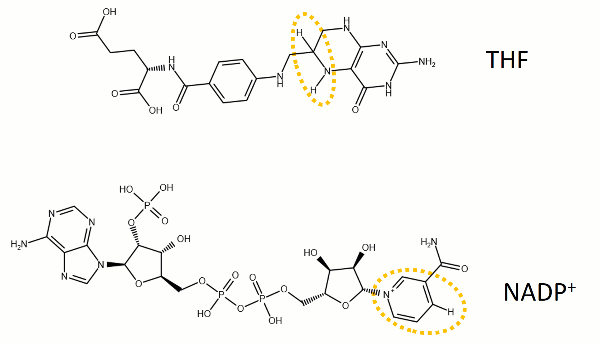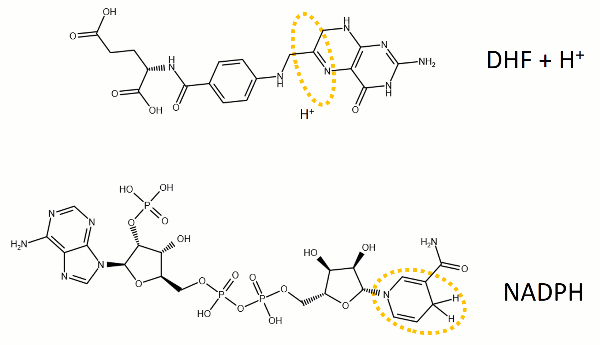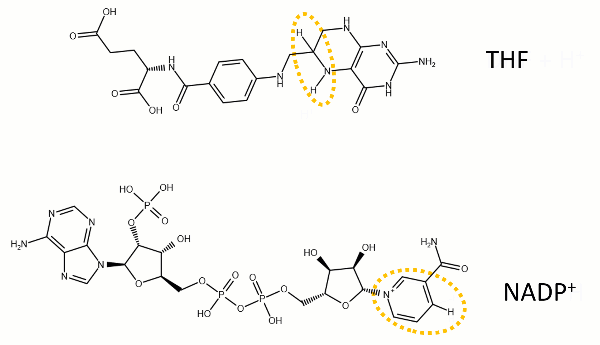
The reaction catalyzed by DHFR reduces a double bond in dihydrofolate (DHF) to form tetrahydrofolate (THF) by transfering a hydride from nicotinamide adenine dinucleotide phosphate (NADPH)
Dihydrofolate reductase (DHFR, 1.5.1.3 [2]) is an enzyme which uses the co-factor NADPH as electron donor. It catalyzes the reduction of dihydrofolic acid (DHF) to tetrahydrofolic acid (THF) as NADPH is oxidized to NADP+. The mammalian enzymes also accept folic acid as a substrate, reducing it to THF. This allows the use of folic acid, which is easier to synthesize than DHF or THF, to fortify food.[7][8]. Some bacterial enzymes also accept folic acid as a substrate [9] but it acts as a competitive inhibitor in the E. coli enzyme.
The folate is a form of the essential vitamin B9. Folate is not part of our natural diet (it contains dihydrofolate and tetrahydrofolate, sometimes as a poly-glutamate conjugate) but is bioavailable and simpler to synthesize.
Relevance
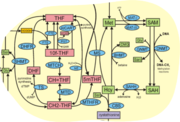
Tetrahydrofolate (THF) is an essential cofactor of one-carbon metabolism[10][11].Structure and Function
| |||||||||||
See also
- An overview of pathways involving folate and S-adenosyl methioine: One-carbon metabolism
- Another page on DHFR: Molecular Playground/DHFR
- Fighting malaria with antifolates" Malarial Dihydrofolate Reductase as Drug Target.
- Overview of enzymes targeted in cancer therapy: Cancer.
3D Structures of Dihydrofolate reductase
Dihydrofolate reductase 3D structures
Acknowledgements
This page was revised as part of the Spring 2022 Biochemistry II course at WSU. Thanks go to Kia, Anna, Shaylie and Michael for helpful suggestions to improve the page.
References
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Karsten Theis, Alexander Berchansky, Joel L. Sussman, Tzvia Selzer, Jaime Prilusky, Eric Martz, Eran Hodis, David Canner