User:George G. Papadeas/Sandbox VKOR
From Proteopedia
< User:George G. Papadeas(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
References: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | References: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
| - | == Introduction== | ||
=== Biological Role === | === Biological Role === | ||
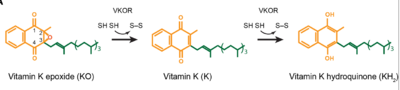
| - | <scene name='90/906893/Vkor_structure/1'>Vitamin K epoxide reductase</scene> (VKOR) is a reducing enzyme composed of 4-helices that spans the endoplasmic reticulum as a transmembrane protein <ref>DOI 10.1126</ref>. Its enzymatic role is reducing <scene name='90/906893/Vkor_with_ko/1'>vitamin K epoxide</scene> (KO) to Vitamin K Hydroquinone (KH2) <ref>DOI 10.1021</ref> (Figure 1). The mechanism first occurs through the binding KO and using two cysteine residues to reduce KO into [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K]. Then, a second pair of cysteine residues will reduce Vitamin K into the final product, KH2 (Figure 1). One of VKORs primary roles is to assist in blood coagulation through this KH2 regeneration mechanism.[[Image:VKOR_mechanism_2D.png|400 px|right|thumb|Figure 1. Mechanism of KO | + | <scene name='90/906893/Vkor_structure/1'>Vitamin K epoxide reductase</scene> (VKOR) is a reducing enzyme composed of 4-helices that spans the endoplasmic reticulum as a transmembrane protein<ref>DOI 10.1126/science.abc5667</ref>. Its enzymatic role is reducing <scene name='90/906893/Vkor_with_ko/1'>vitamin K epoxide</scene> (KO) to Vitamin K Hydroquinone (KH2)<ref>DOI 10.1021/bi700527j</ref> (Figure 1). The mechanism first occurs through the binding KO and using two cysteine residues to reduce KO into [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K]. Then, a second pair of cysteine residues will reduce Vitamin K into the final product, KH2 (Figure 1). One of VKORs primary roles is to assist in blood coagulation through this KH2 regeneration mechanism.[[Image:VKOR_mechanism_2D.png|400 px|right|thumb|Figure 1. Mechanism of KO reduction into KH2.]] With Vitamin K as a cofactor, the [https://www.britannica.com/science/bleeding/The-extrinsic-pathway-of-blood-coagulation#ref64617 γ-carboxylase] enzyme will enact post-translational modification on KH2, oxidizing it back to KO <ref>DOI 10.1074/jbc.RA120.015401</ref>. The oxidation of KH2 by γ-carboxylase is coupled with the carboxylation of a glutamate residue to form γ-carboxyglutamate. The coupling of this oxidation and carboxylation will activate several clotting factors in the coagulation cascade. |
=== Author's Notes === | === Author's Notes === | ||
| - | Structural characterization of VKOR has been difficult due to its in vitro instability. Recently, a series of atomic structures have been determined utilizing anticoagulant stabilization and VKOR-like | + | Structural characterization of VKOR has been difficult due to its in vitro instability. Recently, a series of atomic structures have been determined utilizing anticoagulant stabilization and VKOR-like homologs<ref>DOI 10.1126/science.abc5667</ref>. Crystal structures of VKOR were captured with a bound substrate (KO) or vitamin K antagonist (VKA)<ref>DOI 10.1126/science.abc5667</ref>. VKA substrates utilized were anticoagulants, namely [https://en.wikipedia.org/wiki/Warfarin Warfarin], [https://en.wikipedia.org/wiki/Brodifacoum Brodifacoum], [https://en.wikipedia.org/wiki/Phenindione Phenindione], and [https://en.wikipedia.org/wiki/Chlorophacinone Chlorophacinone]. Second, VKOR-like homologs were utilized to aid in structure classification. Homologs refer to specific cysteine residues that have been mutated to serine to facilitate capturing a stable conformation state. Homologs were mainly isolated from human VKOR with some isolated from the pufferfish ''Takifugu rubripes''. Homologs were also tagged at the N and C with a superfolder [https://en.wikipedia.org/wiki/Green_fluorescent_protein#Applications Green Fluorescent Protein](sfGFP)<ref>DOI 10.1126/science.abc5667</ref>. The [https://proteopedia.org/wiki/index.php/3ed8 sfGFP] provides in vitro stability, a scaffold for crystallization, and facilitates in structure determination. Also, the sfGFP induces states of catalytic activity and potential inhibition for VKOR homologs. For the purpose of this report, all of the structures used have been processed to remove the sfGFP at the south end of VKOR as sfGFP served no purpose in function of the enzyme. This removal allowed for the residue numbering to be reassigned and more closely replicate the human VKOR. |
== Structural Highlights== | == Structural Highlights== | ||
| Line 17: | Line 16: | ||
=== Active Site === | === Active Site === | ||
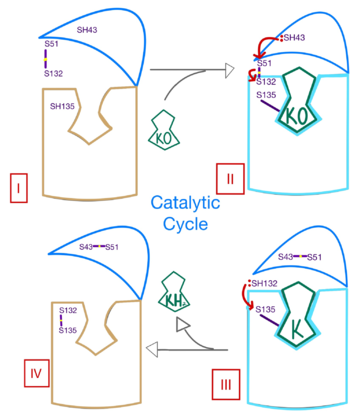
| - | Within the four transmembrane helices lies the <scene name='90/906893/Binding_pocket/1'>binding pocket</scene>. The binding pocket | + | Within the four transmembrane helices lies the <scene name='90/906893/Binding_pocket/1'>binding pocket</scene>. The binding pocket holds two <scene name='90/906893/Active_site/7'>two hydrophilic residues</scene> active site residues, N80 and Y139, that interact with the substrate. N80 and Y139 are surrounded by a <scene name='90/906893/Hydrophobic/2'>hydrophobic region</scene> that provides specificity to the region. The hydrophilic residues hydrogen bond to the substrate, providing recognition and increasing specificity. The <scene name='90/906893/Disulfide_-_132/1'>C132-C135 disulfide bridge</scene> above the binding pocket provides stabilization when a substrate is bound. This bridge provides increased stability for the binding site as it interacts with and binds substrates or inhibitors. The hydrophilic residues provide <scene name='90/906893/K_hbonds/1'>hydrogen bonds</scene> when interacting with substrates for specificity and recognition. Upon binding, VKOR will transition into the closed conformation allowing the catalytic mechanism to commence. |
==Catalytic Mechanism of VKOR== | ==Catalytic Mechanism of VKOR== | ||
| Line 34: | Line 33: | ||
=== Inhibition === | === Inhibition === | ||
[[Image:Warfarin.png |400 px| right| thumb | Figure 4. Structure of Warfarin.]] | [[Image:Warfarin.png |400 px| right| thumb | Figure 4. Structure of Warfarin.]] | ||
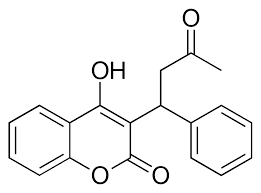
| - | The most common way to treat blood clotting is using the VKOR inhibitor, <scene name='90/904314/Vkor_with_warfarin_bound/1'>warfarin</scene>. [https://en.wikipedia.org/wiki/Warfarin Warfarin] outcompetes KO<ref>PMID: 29261922</ref>, such that Vitamin K cannot be activated to promote coagulation in the blood. Warfarin will enter the binding pocket of VKOR, creating strong <scene name='90/ | + | The most common way to treat blood clotting is using the VKOR inhibitor, <scene name='90/904314/Vkor_with_warfarin_bound/1'>warfarin</scene>. [https://en.wikipedia.org/wiki/Warfarin Warfarin] outcompetes KO<ref>PMID: 29261922</ref>, such that Vitamin K cannot be activated to promote coagulation in the blood. Warfarin will enter the binding pocket of VKOR, creating strong <scene name='90/906893/Vkor_with_warfarin_bound/3'>hydrogen bonds</scene> with the active site residues, N80 and Y139. Mutations of VKOR can lead to warfarin resistance which decreases its anticoagulation effects. Different mutations introduce varying degrees of resistance. These mutations are important to recognize as [https://en.wikipedia.org/wiki/Superwarfarin super-warfarin's] can be overly effective in anticoagulation and become detrimental to blood flow. |
=== Mutations === | === Mutations === | ||
| - | + | Mutations of the <scene name='90/906893/Vkor_with_warfarin_bound/4'>active site residues</scene> can occur within the binding pocket of VKOR. These mutations can be detrimental to the VKOR structure and function<ref>DOI 10.1126/science.abc5667</ref>. Two of the most common mutations occur to residues N80 and Y139 mutating them to <scene name='90/906893/Active_site_mutations/3'>A80 and F139</scene>. The change in polarity of these mutations from polar to nonpolar will cause a decrease in recognition and stabilization due to the inability to provide hydrogen bonds. <scene name='90/906893/Hydrophobic/3'>hydrophobic residues</scene> | |
Current revision
VKOR
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Jin DY, Tie JK, Stafford DW. The conversion of vitamin K epoxide to vitamin K quinone and vitamin K quinone to vitamin K hydroquinone uses the same active site cysteines. Biochemistry. 2007 Jun 19;46(24):7279-83. doi: 10.1021/bi700527j. Epub 2007 May, 25. PMID:17523679 doi:http://dx.doi.org/10.1021/bi700527j
- ↑ Shen G, Cui W, Cao Q, Gao M, Liu H, Su G, Gross ML, Li W. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. J Biol Chem. 2021 Jan-Jun;296:100145. doi: 10.1074/jbc.RA120.015401. Epub 2020, Dec 10. PMID:33273012 doi:http://dx.doi.org/10.1074/jbc.RA120.015401
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Wang Y, Zhang W, Zhang Y, Yang Y, Sun L, Hu S, Chen J, Zhang C, Zheng Y, Zhen Y, Sun K, Fu C, Yang T, Wang J, Sun J, Wu H, Glasgow WC, Hui R. VKORC1 haplotypes are associated with arterial vascular diseases (stroke, coronary heart disease, and aortic dissection). Circulation. 2006 Mar 28;113(12):1615-21. doi: 10.1161/CIRCULATIONAHA.105.580167., Epub 2006 Mar 20. PMID:16549638 doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.105.580167
- ↑ Elshaikh AO, Shah L, Joy Mathew C, Lee R, Jose MT, Cancarevic I. Influence of Vitamin K on Bone Mineral Density and Osteoporosis. Cureus. 2020 Oct 5;12(10):e10816. doi: 10.7759/cureus.10816. PMID:33173624 doi:http://dx.doi.org/10.7759/cureus.10816
- ↑ Patel S, Singh R, Preuss CV, Patel N. Warfarin PMID:29261922
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667