User:George G. Papadeas/Sandbox VKOR
From Proteopedia
< User:George G. Papadeas(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 16: | Line 16: | ||
=== Active Site === | === Active Site === | ||
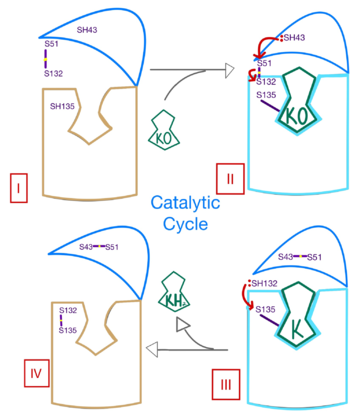
| - | Within the four transmembrane helices lies the <scene name='90/906893/Binding_pocket/1'>binding pocket</scene>. The binding pocket | + | Within the four transmembrane helices lies the <scene name='90/906893/Binding_pocket/1'>binding pocket</scene>. The binding pocket holds two <scene name='90/906893/Active_site/7'>two hydrophilic residues</scene> active site residues, N80 and Y139, that interact with the substrate. N80 and Y139 are surrounded by a <scene name='90/906893/Hydrophobic/2'>hydrophobic region</scene> that provides specificity to the region. The hydrophilic residues hydrogen bond to the substrate, providing recognition and increasing specificity. The <scene name='90/906893/Disulfide_-_132/1'>C132-C135 disulfide bridge</scene> above the binding pocket provides stabilization when a substrate is bound. This bridge provides increased stability for the binding site as it interacts with and binds substrates or inhibitors. The hydrophilic residues provide <scene name='90/906893/K_hbonds/1'>hydrogen bonds</scene> when interacting with substrates for specificity and recognition. Upon binding, VKOR will transition into the closed conformation allowing the catalytic mechanism to commence. |
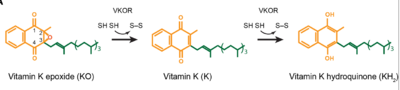
==Catalytic Mechanism of VKOR== | ==Catalytic Mechanism of VKOR== | ||
| Line 33: | Line 33: | ||
=== Inhibition === | === Inhibition === | ||
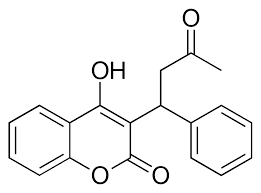
[[Image:Warfarin.png |400 px| right| thumb | Figure 4. Structure of Warfarin.]] | [[Image:Warfarin.png |400 px| right| thumb | Figure 4. Structure of Warfarin.]] | ||
| - | The most common way to treat blood clotting is using the VKOR inhibitor, <scene name='90/904314/Vkor_with_warfarin_bound/1'>warfarin</scene>. [https://en.wikipedia.org/wiki/Warfarin Warfarin] outcompetes KO<ref>PMID: 29261922</ref>, such that Vitamin K cannot be activated to promote coagulation in the blood. Warfarin will enter the binding pocket of VKOR, creating strong <scene name='90/ | + | The most common way to treat blood clotting is using the VKOR inhibitor, <scene name='90/904314/Vkor_with_warfarin_bound/1'>warfarin</scene>. [https://en.wikipedia.org/wiki/Warfarin Warfarin] outcompetes KO<ref>PMID: 29261922</ref>, such that Vitamin K cannot be activated to promote coagulation in the blood. Warfarin will enter the binding pocket of VKOR, creating strong <scene name='90/906893/Vkor_with_warfarin_bound/3'>hydrogen bonds</scene> with the active site residues, N80 and Y139. Mutations of VKOR can lead to warfarin resistance which decreases its anticoagulation effects. Different mutations introduce varying degrees of resistance. These mutations are important to recognize as [https://en.wikipedia.org/wiki/Superwarfarin super-warfarin's] can be overly effective in anticoagulation and become detrimental to blood flow. |
=== Mutations === | === Mutations === | ||
| - | + | Mutations of the <scene name='90/906893/Vkor_with_warfarin_bound/4'>active site residues</scene> can occur within the binding pocket of VKOR. These mutations can be detrimental to the VKOR structure and function<ref>DOI 10.1126/science.abc5667</ref>. Two of the most common mutations occur to residues N80 and Y139 mutating them to <scene name='90/906893/Active_site_mutations/3'>A80 and F139</scene>. The change in polarity of these mutations from polar to nonpolar will cause a decrease in recognition and stabilization due to the inability to provide hydrogen bonds. <scene name='90/906893/Hydrophobic/3'>hydrophobic residues</scene> | |
Current revision
VKOR
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Jin DY, Tie JK, Stafford DW. The conversion of vitamin K epoxide to vitamin K quinone and vitamin K quinone to vitamin K hydroquinone uses the same active site cysteines. Biochemistry. 2007 Jun 19;46(24):7279-83. doi: 10.1021/bi700527j. Epub 2007 May, 25. PMID:17523679 doi:http://dx.doi.org/10.1021/bi700527j
- ↑ Shen G, Cui W, Cao Q, Gao M, Liu H, Su G, Gross ML, Li W. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. J Biol Chem. 2021 Jan-Jun;296:100145. doi: 10.1074/jbc.RA120.015401. Epub 2020, Dec 10. PMID:33273012 doi:http://dx.doi.org/10.1074/jbc.RA120.015401
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Wang Y, Zhang W, Zhang Y, Yang Y, Sun L, Hu S, Chen J, Zhang C, Zheng Y, Zhen Y, Sun K, Fu C, Yang T, Wang J, Sun J, Wu H, Glasgow WC, Hui R. VKORC1 haplotypes are associated with arterial vascular diseases (stroke, coronary heart disease, and aortic dissection). Circulation. 2006 Mar 28;113(12):1615-21. doi: 10.1161/CIRCULATIONAHA.105.580167., Epub 2006 Mar 20. PMID:16549638 doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.105.580167
- ↑ Elshaikh AO, Shah L, Joy Mathew C, Lee R, Jose MT, Cancarevic I. Influence of Vitamin K on Bone Mineral Density and Osteoporosis. Cureus. 2020 Oct 5;12(10):e10816. doi: 10.7759/cureus.10816. PMID:33173624 doi:http://dx.doi.org/10.7759/cureus.10816
- ↑ Patel S, Singh R, Preuss CV, Patel N. Warfarin PMID:29261922
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667