Sandbox Reserved 1719
From Proteopedia
(Difference between revisions)
| (29 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
= Human Itch G-Coupled Protein Receptors = | = Human Itch G-Coupled Protein Receptors = | ||
<StructureSection load='7s8l' size='350' side='right' caption='Cryo-EM structure of Gq coupled MRGPRX2.' scene='90/904324/Mrgprx2/2'> | <StructureSection load='7s8l' size='350' side='right' caption='Cryo-EM structure of Gq coupled MRGPRX2.' scene='90/904324/Mrgprx2/2'> | ||
| + | [[Image:Structure_overview_of_Mx_with_membrane.PNG|500px|right|thumb|'''Figure 1.''' Positioning of MRGPRX2 in plasma membrane.]] | ||
| - | [[Image:Structure_overview_of_Mx_with_membrane.PNG|500px|right|thumb|'''Figure 1.''' Location of MRGPRX2 in plasma membrane.]] | ||
= Introduction = | = Introduction = | ||
[https://proteopedia.org/wiki/index.php/G_protein-coupled_receptors G protein-coupled receptors] (GPCRs) are the largest class of integral membrane proteins divided into five families; the [https://proteopedia.org/wiki/index.php/Sandbox_Reserved_895 rhodopsin family (class A)], the [https://proteopedia.org/wiki/index.php/4ers secretin family (class B)], the [https://proteopedia.org/wiki/index.php/6wiv glutamate family (class C)], the [https://proteopedia.org/wiki/index.php/6bd4 frizzled/taste family (class F)], and the [https://en.wikipedia.org/wiki/Adhesion_G_protein-coupled_receptor adhesion family].<ref name= "Zhang 2006"/><ref>DOI: 10.1210/me.2009-0473</ref><ref name="Zhang 2015">DOI 10.14348/molcells.2015.0263</ref> GPCRs promote signal transduction activated by a variety of stimuli by undergoing a transmembrane domain conformational change once ligand binding to the N-terminus occurs. This allows for the transduction of a signal to a coupled, heterotrimeric G protein, which then dictates whether an intracellular signaling pathway will be initiated or inhibited.<ref name= "Zhang 2006">DOI 10.1371/journal.pcbi.0020013</ref><ref name= "Zhang 2015"/> | [https://proteopedia.org/wiki/index.php/G_protein-coupled_receptors G protein-coupled receptors] (GPCRs) are the largest class of integral membrane proteins divided into five families; the [https://proteopedia.org/wiki/index.php/Sandbox_Reserved_895 rhodopsin family (class A)], the [https://proteopedia.org/wiki/index.php/4ers secretin family (class B)], the [https://proteopedia.org/wiki/index.php/6wiv glutamate family (class C)], the [https://proteopedia.org/wiki/index.php/6bd4 frizzled/taste family (class F)], and the [https://en.wikipedia.org/wiki/Adhesion_G_protein-coupled_receptor adhesion family].<ref name= "Zhang 2006"/><ref>DOI: 10.1210/me.2009-0473</ref><ref name="Zhang 2015">DOI 10.14348/molcells.2015.0263</ref> GPCRs promote signal transduction activated by a variety of stimuli by undergoing a transmembrane domain conformational change once ligand binding to the N-terminus occurs. This allows for the transduction of a signal to a coupled, heterotrimeric G protein, which then dictates whether an intracellular signaling pathway will be initiated or inhibited.<ref name= "Zhang 2006">DOI 10.1371/journal.pcbi.0020013</ref><ref name= "Zhang 2015"/> | ||
| Line 32: | Line 32: | ||
</jmolButton> | </jmolButton> | ||
</jmol> | </jmol> | ||
| - | [[Image:Electro_map_with_cortistatin.png|400px|center|thumb|'''Figure 2.''' Electrostatic surface of MRGPRX2 ligand binding pocket with cortistatin-14]] | + | [[Image:Electro_map_with_cortistatin.png|400px|center|thumb|'''Figure 2.''' Electrostatic surface of MRGPRX2 ligand binding pocket with cortistatin-14. Red indicating regions of negative charge. Blue indicating regions of positive charge. White indicating hydrophobic regions.]] |
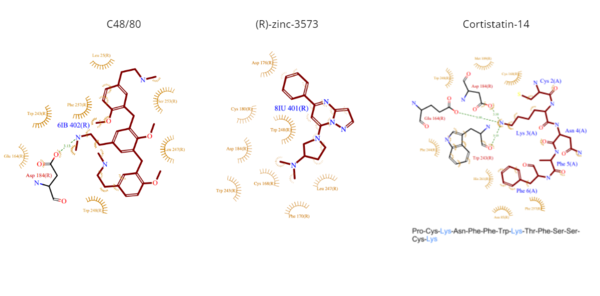
==== Sub-pocket 1 ==== | ==== Sub-pocket 1 ==== | ||
| Line 47: | Line 47: | ||
*<scene name='90/904324/Rzinc3573/7'>(R)-zinc-3573</scene> | *<scene name='90/904324/Rzinc3573/7'>(R)-zinc-3573</scene> | ||
| - | **(R)-zinc-3573 is | + | **(R)-zinc-3573 is a synthetic agonist that binds to MRGPRX2 when it is associated with a G<sub>i</sub> or G<sub>q</sub> protein.<ref name="Can">DOI: 10.1038/s41586-021-04126-6</ref> This agonist is a small cationic molecule that forms largely ionic interactions with the negatively-charged sub-pocket 1 and has no interactions with sub-pocket 2 ('''Figure 4''').<ref name="Can"/> (R)-zinc-3573 forms hydrogen bonds and hydrophobic interactions with Asp184 and Glu164 of sub-pocket 1.<ref name="Can"/><ref>DOI: 10.1038/nchembio.2334</ref> |
*<scene name='90/904324/Cortistatin-14/6'>Cortistatin-14</scene> | *<scene name='90/904324/Cortistatin-14/6'>Cortistatin-14</scene> | ||
| Line 62: | Line 62: | ||
</jmol> | </jmol> | ||
| - | [[Image:Comparison_of_binding_depth.PNG|500px| | + | [[Image:Comparison_of_binding_depth.PNG|500px|right|thumb|'''Figure 5.''' Comparison of the depth of ligand interactions in MRGPRX2 (blue) and 5HT2AR (purple) shown with toggle switch.]] |
| - | + | ||
=== Toggle switch === | === Toggle switch === | ||
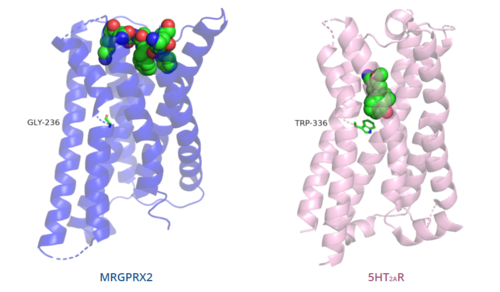
The conserved <scene name='90/904324/5ht2a_toggle_switch/3'>toggle switch</scene> of class A GPCRs functions to activate or inhibit the transduction of the signaling cascade. Typical class A GPCRs contain the conserved ‘toggle switch’ Trp336. In MRGPRX2, this residue is replaced by <scene name='90/904324/Toggle_switch/7'>Gly236</scene>.<ref name="Can"/> By substituting the larger Trp residue with a smaller Gly residue, TM6 is shifted closer to TM3 on the extracellular side of the membrane and contributes to the more tightly packed and shallow binding pocket as compared to other canonical structures. The occluded binding pocket contributes the surface level binding depicted in '''Figure 5''', and allows for a greater variety of ligands interactions with MRGPRX2 as compared to other class A GPCRs, such as [https://proteopedia.org/wiki/index.php/5-hydroxytryptamine_receptor 5-HT<sub>2A</sub>R], [https://proteopedia.org/wiki/index.php/Adrenergic_receptor A<sub>2A</sub>R], and [https://proteopedia.org/wiki/index.php/Beta-2_Adrenergic_Receptor β<sub>2</sub>AR].<ref name="Can"/> | The conserved <scene name='90/904324/5ht2a_toggle_switch/3'>toggle switch</scene> of class A GPCRs functions to activate or inhibit the transduction of the signaling cascade. Typical class A GPCRs contain the conserved ‘toggle switch’ Trp336. In MRGPRX2, this residue is replaced by <scene name='90/904324/Toggle_switch/7'>Gly236</scene>.<ref name="Can"/> By substituting the larger Trp residue with a smaller Gly residue, TM6 is shifted closer to TM3 on the extracellular side of the membrane and contributes to the more tightly packed and shallow binding pocket as compared to other canonical structures. The occluded binding pocket contributes the surface level binding depicted in '''Figure 5''', and allows for a greater variety of ligands interactions with MRGPRX2 as compared to other class A GPCRs, such as [https://proteopedia.org/wiki/index.php/5-hydroxytryptamine_receptor 5-HT<sub>2A</sub>R], [https://proteopedia.org/wiki/index.php/Adrenergic_receptor A<sub>2A</sub>R], and [https://proteopedia.org/wiki/index.php/Beta-2_Adrenergic_Receptor β<sub>2</sub>AR].<ref name="Can"/> | ||
| Line 73: | Line 72: | ||
In MRGPRX2, the PIF motif is changed to a <scene name='90/904324/Pifllf_motif/5'>LLF motif</scene>, in which the residues are shifted down, so that the specific amino acid sequence is not conserved, but instead residues are conserved at distances that allow them to interact.<ref name="Can"/> Specifically, the residues that make up TM5 have shifted down two residues making Leu194 analogous to the position of Pro in other GPCRs. However, one key difference is that Leu194 is positioned closer (5.48Å) than the conserved distance (5.50Å).<ref name="Can"/> Leu194 is involved in this motif, despite the closer positioning, because the residue that is at the canonical distance is angled away from the TM3-TM6 interface, so it is unable to interact.<ref name="Can"/> Compared with other structures, such as [https://proteopedia.org/wiki/index.php/5-hydroxytryptamine_receptor 5-HT<sub>2A</sub>R], [https://proteopedia.org/wiki/index.php/Adrenergic_receptor A<sub>2A</sub>R], and [https://proteopedia.org/wiki/index.php/Beta-2_Adrenergic_Receptor β<sub>2</sub>AR], the TM6 helix of MRGPRX2 is closer to the TM3 helix due to the shift in residues, which accounts for the tighter packing of the transmembrane domain helices<ref name="Can"/>, thereby contributing to surface level ligand interactions. | In MRGPRX2, the PIF motif is changed to a <scene name='90/904324/Pifllf_motif/5'>LLF motif</scene>, in which the residues are shifted down, so that the specific amino acid sequence is not conserved, but instead residues are conserved at distances that allow them to interact.<ref name="Can"/> Specifically, the residues that make up TM5 have shifted down two residues making Leu194 analogous to the position of Pro in other GPCRs. However, one key difference is that Leu194 is positioned closer (5.48Å) than the conserved distance (5.50Å).<ref name="Can"/> Leu194 is involved in this motif, despite the closer positioning, because the residue that is at the canonical distance is angled away from the TM3-TM6 interface, so it is unable to interact.<ref name="Can"/> Compared with other structures, such as [https://proteopedia.org/wiki/index.php/5-hydroxytryptamine_receptor 5-HT<sub>2A</sub>R], [https://proteopedia.org/wiki/index.php/Adrenergic_receptor A<sub>2A</sub>R], and [https://proteopedia.org/wiki/index.php/Beta-2_Adrenergic_Receptor β<sub>2</sub>AR], the TM6 helix of MRGPRX2 is closer to the TM3 helix due to the shift in residues, which accounts for the tighter packing of the transmembrane domain helices<ref name="Can"/>, thereby contributing to surface level ligand interactions. | ||
| + | [[Image:B2AR_motif.png|500px|right|thumb|'''Figure 6.''' Comparison of ERC motif in MRGPRX2 (blue) and conserved DRY motif in β<sub>2</sub>AR (green).]] | ||
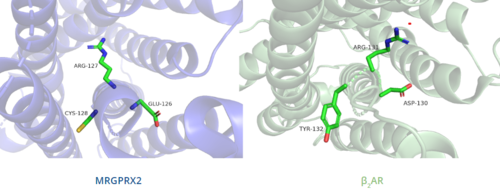
=== DRY/ERC motif === | === DRY/ERC motif === | ||
The majority of class A GPCRs have a [https://proteopedia.org/wiki/index.php/A_Physical_Model_of_the_%CE%B22-Adrenergic_Receptor#conserved%20DRY%20motif conserved E/DRY motif] that is responsible for creating salt bridges that maintain the inactive conformation of the receptor until ligand binding.<ref name="Can"/> | The majority of class A GPCRs have a [https://proteopedia.org/wiki/index.php/A_Physical_Model_of_the_%CE%B22-Adrenergic_Receptor#conserved%20DRY%20motif conserved E/DRY motif] that is responsible for creating salt bridges that maintain the inactive conformation of the receptor until ligand binding.<ref name="Can"/> | ||
In contrast, '''Figure 6''' shows MRGPRX2 containing an <scene name='90/904324/Erc_motif/3'>ERC motif</scene> in place of the E/DRY motif, which replaces Tyr174 with Cys128.<ref name="Can"/> This replacement alters the spatial organization of the helices due to the replacement of the larger Tyr residue with the smaller Cys residue, thereby condensing the helices.<ref name="Can"/> The condensed spatial organization in MRGPRX2 accounts for the less significant conformational change observed once a ligand binds to the receptor.<ref name="Can"/> | In contrast, '''Figure 6''' shows MRGPRX2 containing an <scene name='90/904324/Erc_motif/3'>ERC motif</scene> in place of the E/DRY motif, which replaces Tyr174 with Cys128.<ref name="Can"/> This replacement alters the spatial organization of the helices due to the replacement of the larger Tyr residue with the smaller Cys residue, thereby condensing the helices.<ref name="Can"/> The condensed spatial organization in MRGPRX2 accounts for the less significant conformational change observed once a ligand binds to the receptor.<ref name="Can"/> | ||
| - | [[Image:B2AR_motif.png|500px|center|thumb|'''Figure 6.''' Comparison of ERC motif in MRGPRX2 (blue) and conserved DRY motif in β<sub>2</sub>AR (green).]] | ||
| + | [[Image:Disulfide_bond_comparison.png|500px|left|thumb|'''Figure 7.''' Comparison of disulfide bond location in MRGPRX2 (blue) and 5HT2AR (purple).]] | ||
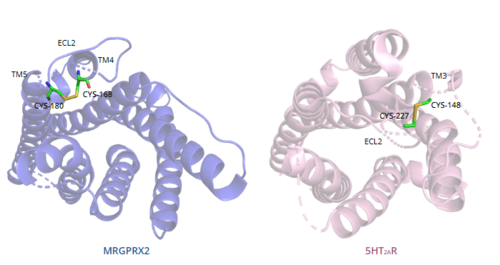
=== Disulfide bonds === | === Disulfide bonds === | ||
In general, class A GPCRs have a <scene name='90/904324/5ht2a/4'>conserved disulfide bond</scene> between TM3 and ECL2.<ref name="Can"/> In contrast, '''Figure 7''' shows the location of the MRGPRX2 <scene name='90/904324/Mrgprx2_disulfide_bonds/5'>disulfide bond</scene> between Cys168 of TM4 and Cys180 of TM5, which structurally flips ECL2 to the top of TM4 and TM5.<ref name="Can"/> This creates the wide ligand-binding surface of MRGPRX2 that contributes to surface level binding and allows diverse ligand interactions.<ref name="Can"/> | In general, class A GPCRs have a <scene name='90/904324/5ht2a/4'>conserved disulfide bond</scene> between TM3 and ECL2.<ref name="Can"/> In contrast, '''Figure 7''' shows the location of the MRGPRX2 <scene name='90/904324/Mrgprx2_disulfide_bonds/5'>disulfide bond</scene> between Cys168 of TM4 and Cys180 of TM5, which structurally flips ECL2 to the top of TM4 and TM5.<ref name="Can"/> This creates the wide ligand-binding surface of MRGPRX2 that contributes to surface level binding and allows diverse ligand interactions.<ref name="Can"/> | ||
| - | [[Image:Disulfide_bond_comparison.png|500px|center|thumb|'''Figure 7.''' Comparison of disulfide bond location in MRGPRX2 (blue) and 5HT2AR (purple).]] | ||
=== Sodium binding site === | === Sodium binding site === | ||
| Line 92: | Line 91: | ||
G<sub>αq</sub> and G<sub>αi</sub> are proteins comprised of 359 amino acid residues, with varying sequences, that both contain a helical domain and a GTPase binding domain.<ref name= "Kamato">DOI: 10.3389/fcvm.2015.00014</ref> The GTPase binding domain is responsible for the hydrolysis of GTP as well as the binding of the β and γ subunits that form the trimeric protein structure. The helical domain contains six α helices, which are responsible for the binding of the G-protein to the coupled receptor.<ref name="Kamato"/> | G<sub>αq</sub> and G<sub>αi</sub> are proteins comprised of 359 amino acid residues, with varying sequences, that both contain a helical domain and a GTPase binding domain.<ref name= "Kamato">DOI: 10.3389/fcvm.2015.00014</ref> The GTPase binding domain is responsible for the hydrolysis of GTP as well as the binding of the β and γ subunits that form the trimeric protein structure. The helical domain contains six α helices, which are responsible for the binding of the G-protein to the coupled receptor.<ref name="Kamato"/> | ||
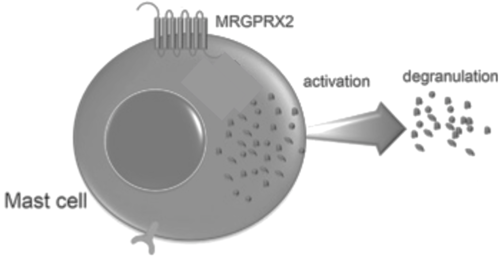
| + | [[Image:Screen_Shot_2022-04-18_at_3.33.37_PM.png|500px|right|thumb|'''Figure 8.''' Cellular response of mast cell upon activation of MRGPRX2.]] | ||
The conformations of the G-proteins vary based on their association with a particular membrane receptor due to interactions between the amino acids in the N-terminus of the α subunit and the C-terminus of the receptor.<ref name="Kamato"/> | The conformations of the G-proteins vary based on their association with a particular membrane receptor due to interactions between the amino acids in the N-terminus of the α subunit and the C-terminus of the receptor.<ref name="Kamato"/> | ||
| - | [[Image:Screen_Shot_2022-04-18_at_3.33.37_PM.png|500px|right|thumb|'''Figure 8.''' Cellular response of mast cell upon activation of MRGPRX2.]] | ||
| - | |||
| - | |||
Binding to the extracellular N-terminus domain triggers a transmembrane conformation change of MRGPRX2, which demonstrates a less significant change when compared to other class A GPCRs due to the surface level binding of the ligand to MRGPRX2.<ref name="Can"/> Once ligand binding and the conformational change to the active state have taken place, the signal is relayed to the α-subunit of the heterotrimeric G-protein.<ref name="nelson"/> The α-subunit will then exchange a GDP for GTP to initiate the dissociation of the α, β, and γ subunits.<ref name="nelson"/> During this dissociation, the α-subunit is able to travel away from the receptor in the plane of the membrane to bind to downstream effectors to produce a cellular response.<ref name="nelson"/> | Binding to the extracellular N-terminus domain triggers a transmembrane conformation change of MRGPRX2, which demonstrates a less significant change when compared to other class A GPCRs due to the surface level binding of the ligand to MRGPRX2.<ref name="Can"/> Once ligand binding and the conformational change to the active state have taken place, the signal is relayed to the α-subunit of the heterotrimeric G-protein.<ref name="nelson"/> The α-subunit will then exchange a GDP for GTP to initiate the dissociation of the α, β, and γ subunits.<ref name="nelson"/> During this dissociation, the α-subunit is able to travel away from the receptor in the plane of the membrane to bind to downstream effectors to produce a cellular response.<ref name="nelson"/> | ||
| - | |||
| - | |||
= Clinical Relevance = | = Clinical Relevance = | ||
| - | The discovery of <scene name='90/904324/Mrgprx2/5'>MRGPRX2</scene>-mediated allergic reactions has provided additional insight into anaphylactic and allergic-type reactions to medications. The MRGPRX2 mechanism allows for the triggering of mast | + | The discovery of <scene name='90/904324/Mrgprx2/5'>MRGPRX2</scene>-mediated allergic reactions has provided additional insight into anaphylactic and allergic-type reactions to medications. The MRGPRX2 mechanism allows for the triggering of [https://proteopedia.org/wiki/index.php/1klt mast cell] granulation without immune priming due to the fact that it bypasses the previously known antibody-mediated, or IgE-mediated pathway.<ref name="porebski">DOI: 10.3389/fimmu.2018.03027</ref><ref name="ben"/> |
The activation of the MRGPRX2-mediated pathway has been labeled as “pseudo-allergic” events, so as to separate them from true allergic reactions.<ref name="ben"/> Due to this distinction, there is now the possibility that side effects that were previously attributed to IgE-mediated reactions, may have actually been caused by MRGPRX2-mediated reactions.<ref name="ben"/> | The activation of the MRGPRX2-mediated pathway has been labeled as “pseudo-allergic” events, so as to separate them from true allergic reactions.<ref name="ben"/> Due to this distinction, there is now the possibility that side effects that were previously attributed to IgE-mediated reactions, may have actually been caused by MRGPRX2-mediated reactions.<ref name="ben"/> | ||
| - | In general, MRGPRX2-mediated anaphylactic responses occur more quickly than IgE-mediated responses, but the responses also tended to be more transient.<ref name="porebski"/><ref name="ben"/> Common commercial drugs, like icatibant and cetrorelix, as well as neuromuscular blocking agents activate mast cells through the MRGPRX2 pathway. Furthermore, due to the wide and shallow binding pocket of MRGPRX2, there is a wide range of molecules, and thus medications, that can possibly bind and activate this mechanism.<ref name="ben">DOI: 10.3389/fimmu.2021.676354</ref> The majority of these medications have a net positive charge and carry cationic groups. | + | In general, MRGPRX2-mediated anaphylactic responses occur more quickly than IgE-mediated responses, but the responses also tended to be more transient.<ref name="porebski"/><ref name="ben"/> Common commercial drugs, like [https://en.wikipedia.org/wiki/Icatibant icatibant] and [https://en.wikipedia.org/wiki/Cetrorelix cetrorelix], as well as neuromuscular blocking agents activate mast cells through the MRGPRX2 pathway. Furthermore, due to the wide and shallow binding pocket of MRGPRX2, there is a wide range of molecules, and thus medications, that can possibly bind and activate this mechanism.<ref name="ben">DOI: 10.3389/fimmu.2021.676354</ref> The majority of these medications have a net positive charge and carry cationic groups. |
Many mutations also affect the actions of MRGPRX2. For example, a single residue mutation in sub-pocket 1 (Glu164Arg) prevented interactions between the receptor and ligands like C48/80.<ref name="porebski"/> In addition, single nucleotide polymorphisms (SNPs) have been linked to many variations of MRGPRX2 which predispose patients to hyperactivation of the receptors. Two of the most common SNPs are Asn62Thr which affects the cytoplasmic domain and Asn16His which affects the extracellular domain.<ref name="porebski"/> These mutations have been theorized to potentially protect patients from drug-induced mast cell degranulation and hypersensitivity reactions. | Many mutations also affect the actions of MRGPRX2. For example, a single residue mutation in sub-pocket 1 (Glu164Arg) prevented interactions between the receptor and ligands like C48/80.<ref name="porebski"/> In addition, single nucleotide polymorphisms (SNPs) have been linked to many variations of MRGPRX2 which predispose patients to hyperactivation of the receptors. Two of the most common SNPs are Asn62Thr which affects the cytoplasmic domain and Asn16His which affects the extracellular domain.<ref name="porebski"/> These mutations have been theorized to potentially protect patients from drug-induced mast cell degranulation and hypersensitivity reactions. | ||
Current revision
Human Itch G-Coupled Protein Receptors
| |||||||||||