User:Glauco O. Gavioli Ferreira/Sandbox 1
From Proteopedia
< User:Glauco O. Gavioli Ferreira(Difference between revisions)
| (31 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
=SerpinB5 (Maspin)= | =SerpinB5 (Maspin)= | ||
<StructureSection load='1wz9' size='350' side='right' caption='The 2.1 A structure of a tumour suppressing serpin called maspin (PDB code [[1wz9]]).' scene='91/915218/Maspin/1'> | <StructureSection load='1wz9' size='350' side='right' caption='The 2.1 A structure of a tumour suppressing serpin called maspin (PDB code [[1wz9]]).' scene='91/915218/Maspin/1'> | ||
| - | <b>SerpinB5</b>, also known as | + | <b>SerpinB5</b>, also known as "mammary [[serine protease]] inhibitor" (<scene name='91/915218/Maspin/2'>maspin</scene>), is considered a tumor suppressor serpin that does not present itself as a protein inhibitor like others of its own family, the serine protease inhibitor superfamily (serpins). Maspin was first identified in 1994 on mammary tissue and breast cancer cell lines <ref name="numero1">PMID: 17189399</ref>, but it is also known to be expressed on a wide range of cell types and tissues, mainly in epithelial cells, i. e. in prostate, lung, skin, and corneal stromal cells <ref name="numero2">Banias L, Jung I, Gurzu S. Subcellular expression of maspin – from normal tissue to tumor cells. World J Meta-Anal 2019; 7(4): 142-155. doi: https://dx.doi.org/10.13105/wjma.v7.i4.142</ref>. It differs from ordinary serpins once it does not undergo the <b>stressed (S) to relaxed (R) conformation</b> which is a striking feature of other proteins in serpin’s superfamily <ref name="numero1" />. Instead, its G-helix has quite a flexibility, capable of changing the conformation of the protein itself <ref name="P">PMID: 15760906</ref>. |
== Maspin and its superfamily == | == Maspin and its superfamily == | ||
====Serpins==== | ====Serpins==== | ||
| - | + | A [[serpin]] usually inhibit other proteins like serine proteases, caspases and papain-like cysteine proteases. However, some of them do not accomplish an inhibitory role. As an example, some of them function as hormone transporters, molecular chaperones or even as tumor suppressors <ref name="numero3">PMID: 16737556</ref>. Inhibitory serpins are considered “suicide molecules” because they can only be used once <ref name="numero6">PMID: 11057674</ref>. | |
Serpins structure usually contain three ß-sheets (A, B and C) and eight to nine 𝛂-helices (hA-hI) on their structure, and the most important region to interact with their targets is the <scene name='91/915218/Rcl2/1'>reactive center loop (RCL)</scene>.The RCL is usually positioned out of the body of the serpins. When inhibiting proteases, serpins get their RCL cleaved out of the main structure, causing the amino-terminal portion of the RCL to form an additional fourth strand called s4A, once it is inserted into the center of ß-sheet A. This cleavage and modification on the structure of serpin is called the <b>stressed (S) to relaxed (R) transition</b>, in which the protein is in its biologically active state and transitions to a more thermal stable and latent state, respectively <ref name="numero3" />. | Serpins structure usually contain three ß-sheets (A, B and C) and eight to nine 𝛂-helices (hA-hI) on their structure, and the most important region to interact with their targets is the <scene name='91/915218/Rcl2/1'>reactive center loop (RCL)</scene>.The RCL is usually positioned out of the body of the serpins. When inhibiting proteases, serpins get their RCL cleaved out of the main structure, causing the amino-terminal portion of the RCL to form an additional fourth strand called s4A, once it is inserted into the center of ß-sheet A. This cleavage and modification on the structure of serpin is called the <b>stressed (S) to relaxed (R) transition</b>, in which the protein is in its biologically active state and transitions to a more thermal stable and latent state, respectively <ref name="numero3" />. | ||
==== Maspin ==== | ==== Maspin ==== | ||
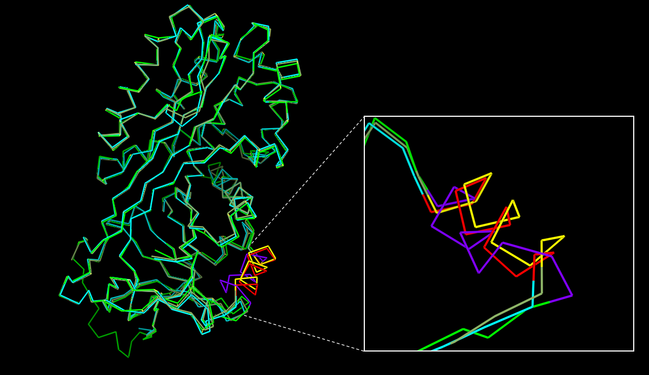
| - | Maspin is a 42 kDa protein <ref name="numero7">PMID: 8290962</ref> and is inserted in clade B of the <b>serpin superfamily</b>, composed of papain-like enzymes and inhibitory serpins that target cytotoxic apoptotic proteases which are working incorrectly <ref name="numero3" />. Differently from other serpins, maspin does not undergo the S to R transition <ref name="numero3" />. Instead, its <scene name='91/915218/G_helix/1'>G-helix</scene> is capable of undergoing a significant conformational change, that means this region of the molecule has some flexibility that allows movement. Accordingly, it is important to mention that studies have demonstrated, by superposing all of the maspin chains, a conformational heterogeneity at and around the G-helix <ref name="P" />. | + | Maspin is a 42 kDa protein <ref name="numero7">PMID: 8290962</ref> and is inserted in clade B of the <b>serpin superfamily</b>, composed of papain-like enzymes and inhibitory serpins that target cytotoxic apoptotic proteases which are working incorrectly <ref name="numero3" />. Differently from other serpins, maspin does not undergo the S to R transition <ref name="numero3" />. Instead, its <scene name='91/915218/G_helix/1'>G-helix</scene> is capable of undergoing a significant conformational change, that means this region of the molecule has some flexibility that allows movement. Accordingly, it is important to mention that studies have demonstrated, by superposing all of the maspin chains, a conformational heterogeneity at and around the G-helix (Fig. 1) <ref name="P" />. |
| - | Also maspin is not limited to a certain cell compartment, once it is found on nucleus, cytoplasm, membrane, and as a secreted protein, according to the cell type and tissue <ref name="numero1" /><ref name="numero2" />. Currently, it is known that the subcellular location of maspin is important for its <b>tumor suppressor activity</b>, and not only its protein levels inside the cell. In the past, there was a controversy about it, once maspin was upregulated in some tumors, while downregulated in others. Then, its translocation to the nucleus was observed and maspin’s nuclear localization was related to its tumor suppressor function <ref name="numero8">PMID: 21502940</ref>. However, contrary to what is expected, it has never been found a nuclear localization sequence (NLS), nuclear export sequence (NES), neither a secretory leader sequence (SLS) on maspin structure <ref name="numero4">PMID: 22752408</ref>. A map of maspin-like proteins is shown in Figure | + | [[Image:Alinhamento focoGhelice.png|thumb|right|649x375px|<b>Fig. 1</b> - Alignment and comparisons of maspin structures. A structural superposition of the 2.1 Å (PDB code [[1wz9]]), a 2.8 Å (PDB code [[1xu8]]) and a closed (PDB code [[1xqg]]) maspin structures. Heterogeneity in and around the G-helix is shown in more details where G-helix from 2.1 Å structure is highlighted in <font color='red'><b>red</b></font>, 2.8 Å structure in <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span> and closed maspin structure in <font color='purple'><b>purple</b></font> (INSPIRED BY doi: http://dx.doi.org/10.1074/jbc.M412043200).]] |
| + | |||
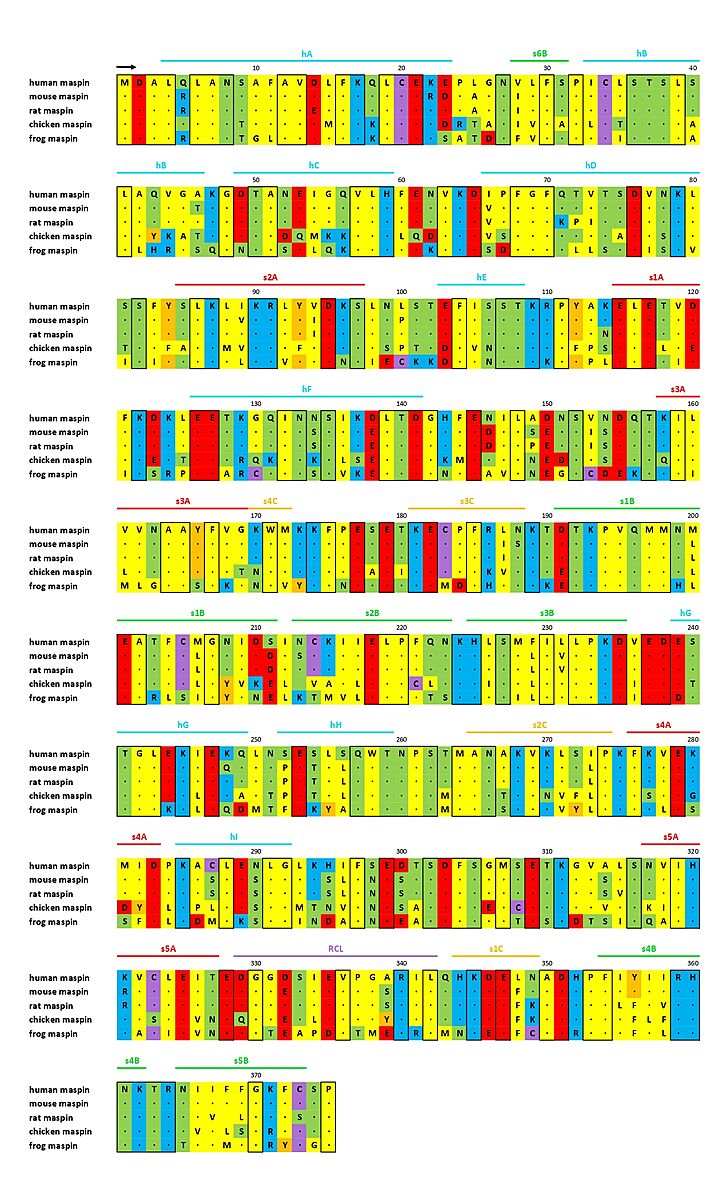
| + | Also maspin is not limited to a certain cell compartment, once it is found on nucleus, cytoplasm, membrane, and as a secreted protein, according to the cell type and tissue <ref name="numero1" /><ref name="numero2" />. Currently, it is known that the subcellular location of maspin is important for its <b>tumor suppressor activity</b>, and not only its protein levels inside the cell. In the past, there was a controversy about it, once maspin was upregulated in some tumors, while downregulated in others. Then, its translocation to the nucleus was observed and maspin’s nuclear localization was related to its tumor suppressor function <ref name="numero8">PMID: 21502940</ref>. However, contrary to what is expected, it has never been found a nuclear localization sequence (NLS), nuclear export sequence (NES), neither a secretory leader sequence (SLS) on maspin structure <ref name="numero4">PMID: 22752408</ref>. A map of maspin-like proteins is shown in Figure 2. | ||
The tumor suppressor function of maspin is probably related to its activities, which are mainly inhibition of cell growth, invasion, tumoral migration, apoptosis stimuli, gene transcription regulation, angiogenesis inhibition <ref name="numero4" /> and prevention of oxidative damage of the proteome <ref name="numero5">PMID: 16049007</ref>. Besides all of these functions, maspin also has an important role in the organization of the epiblast during early embryonic development. However, maspin lacks studies on non-tumoral cell lines, and its role on a normal condition might be different from its activity inside a tumoral lineages <ref name="P" />. | The tumor suppressor function of maspin is probably related to its activities, which are mainly inhibition of cell growth, invasion, tumoral migration, apoptosis stimuli, gene transcription regulation, angiogenesis inhibition <ref name="numero4" /> and prevention of oxidative damage of the proteome <ref name="numero5">PMID: 16049007</ref>. Besides all of these functions, maspin also has an important role in the organization of the epiblast during early embryonic development. However, maspin lacks studies on non-tumoral cell lines, and its role on a normal condition might be different from its activity inside a tumoral lineages <ref name="P" />. | ||
| - | [[Image:Imagem1_mapa.jpg|thumb|center|729x1200px|<b>Fig. | + | [[Image:Imagem1_mapa.jpg|thumb|center|729x1200px|<b>Fig. 2</b> - Sequence alignment of maspin-like proteins. The secondary structure of maspin is marked above the alignment and conserved residues are enclosed in a black box (ADAPTED FROM doi: http://dx.doi.org/10.1074/jbc.M412043200).]] |
| Line 26: | Line 28: | ||
== Function and Structural highlights == | == Function and Structural highlights == | ||
| + | |||
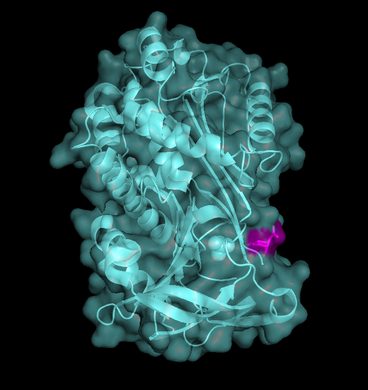
| + | [[Image:SuperficieRCL.png|thumb|left|368x391px|<b>Fig. 3</b> - Maspin's ribbon/cartoon and surfice representation combined (PDB code [[1wz9]]). RCL is highlighted in <font color='fuchsia'><b>pink</b></font>.]] | ||
| + | |||
====RCL==== | ====RCL==== | ||
| - | <scene name='91/915218/Maspinn_c/10'>Maspin structure*</scene> does not differ a lot from other clade B serpins. It has three <scene name='91/915218/Beta-sheets/ | + | <scene name='91/915218/Maspinn_c/10'>Maspin structure*</scene> does not differ a lot from other clade B serpins. It has three <scene name='91/915218/Beta-sheets/5'>ß-sheets</scene>, nine <scene name='91/915218/Alpha-helices/7'>𝛂-helices</scene> and a <scene name='91/915218/Rcl/11'>RCL</scene> (where you can find <font color='slategray'><b>hydrophobic</b></font> and <font color='darkorange'><b>polar</b></font> residues in stick/wireframe representation, but also its <scene name='91/915218/Maspinrcl_sphere/1'>spacefill</scene> and <scene name='91/915218/Maspin_surface/6'>surface</scene> representations, this last one also combined with its ribbon/cartoon representation in Figure 3). The latter is also exposed in ordinary serpins and has a great flexibility. |
Serpins that have mutations within their RCL which interfere with the ability to undergo the S to R conformational change cannot inhibit proteases and maspin’s RCL is the one among serpins that has the most different sequence <ref name="P" /><ref name="numero9">PMID: 15501821</ref><ref name="numero10">PMID: 11435447</ref>. | Serpins that have mutations within their RCL which interfere with the ability to undergo the S to R conformational change cannot inhibit proteases and maspin’s RCL is the one among serpins that has the most different sequence <ref name="P" /><ref name="numero9">PMID: 15501821</ref><ref name="numero10">PMID: 11435447</ref>. | ||
Maspin does not present the conformational switch already discussed and does not have the consensus motif present in other serpins <ref name="P" />. The intact RCL is necessary for maspin’s activity as a tumor suppressor <ref name="numero11">PMID: 7983035</ref>, but there is no rearrangement of this structure, in other words, there is no S to R conformational change <ref name="numero12">PMID: 7797587</ref><ref name="numero13">PMID: 12384513</ref>. Besides that, the RCL alone has been related to cell matrix adhesion and inhibition of cell invasion <ref name="numero14">PMID: 12799381</ref>. | Maspin does not present the conformational switch already discussed and does not have the consensus motif present in other serpins <ref name="P" />. The intact RCL is necessary for maspin’s activity as a tumor suppressor <ref name="numero11">PMID: 7983035</ref>, but there is no rearrangement of this structure, in other words, there is no S to R conformational change <ref name="numero12">PMID: 7797587</ref><ref name="numero13">PMID: 12384513</ref>. Besides that, the RCL alone has been related to cell matrix adhesion and inhibition of cell invasion <ref name="numero14">PMID: 12799381</ref>. | ||
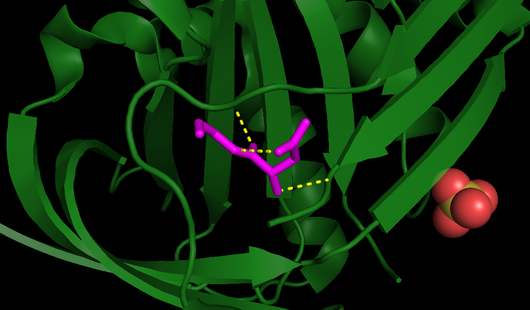
| - | One of the reasons for maspin’s RCL being unable to undergo the conformational switch is its <b>limited flexibility</b>, as it is not flexible like other serpins <ref name="P" />. The RCL of maspin is shorter by four residues and lies closer to the | + | One of the reasons for maspin’s RCL being unable to undergo the conformational switch is its <b>limited flexibility</b>, as it is not flexible like other serpins <ref name="P" />. The RCL of maspin is shorter by four residues and lies closer to the core of the molecule, it is positioned further “back”, in other words closer to the N-terminal, than all of the other known serpin RCL structures. Besides that, the RCL of maspin is also stabilized by bonding interactions with <scene name='91/915218/Betacandrcl/2'>amino acid side chains of the ß-sheet C</scene> (Fig. 4), leading to a more rigid structure. Additionally, the breach, which is presented in other serpins and where the cleaved RCL is inserted, is not seen on maspin <ref name="numero15">PMID: 15501821</ref>. |
| + | |||
| + | [[Image:RCL_lig.png|thumb|right|530x310px|<b>Fig. 4</b> - All RCL bonds, including those responsible for its stability (PDB code [[1wz9]]). RCL is highlighted in <font color='fuchsia'><b>pink</b></font> and all its bonds are displayed as <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span> dashed lines.]] | ||
| + | |||
| + | |||
A curious phenomenon that happens with maspin is the aggregation of <scene name='91/915218/Maspin_dimer/2'>dimers</scene> of tetramers, generating octamers <i>in vitro</i>. There is strong evidence that the hydrophobic residues on the RCL are responsible for the aggregation. The hydrophobic residues Val-336, Ile-341, Leu-342, Pro-337 and adjacent aminoacids present on the RCL are completely exposed to the solvent. Other types of intermolecular interactions, like minor salt links and hydrogen bonds between the s3C and s4C strands of opposing tetramers, also contribute to maintaining the structure, but the main force that results in the octamer are the hydrophobic associations <ref name="numero15" />. Taking into account that the RCL is responsible for functions of cell matrix adhesion and inhibition of cell invasion <ref name="numero14" />, its hydrophobic nature is expected to be functionally important <ref name="numero15" />. | A curious phenomenon that happens with maspin is the aggregation of <scene name='91/915218/Maspin_dimer/2'>dimers</scene> of tetramers, generating octamers <i>in vitro</i>. There is strong evidence that the hydrophobic residues on the RCL are responsible for the aggregation. The hydrophobic residues Val-336, Ile-341, Leu-342, Pro-337 and adjacent aminoacids present on the RCL are completely exposed to the solvent. Other types of intermolecular interactions, like minor salt links and hydrogen bonds between the s3C and s4C strands of opposing tetramers, also contribute to maintaining the structure, but the main force that results in the octamer are the hydrophobic associations <ref name="numero15" />. Taking into account that the RCL is responsible for functions of cell matrix adhesion and inhibition of cell invasion <ref name="numero14" />, its hydrophobic nature is expected to be functionally important <ref name="numero15" />. | ||
Further research is needed to understand if this phenomenon also happens <i>in vivo</i> conditions. | Further research is needed to understand if this phenomenon also happens <i>in vivo</i> conditions. | ||
The RCL might be important for defining the protein subcellular localization. Modification of the Aspartate 346 (D346) by a glutamic acid (E) residue on the <scene name='91/915218/Rclterminals/1'>C-terminal portion of RCL*</scene> in maspin leaded maspin to a dominant nuclear distribution and increased interaction with HDAC1 in multiple cancer cell lines <ref name="numero16">PMID: 24278104</ref>. | The RCL might be important for defining the protein subcellular localization. Modification of the Aspartate 346 (D346) by a glutamic acid (E) residue on the <scene name='91/915218/Rclterminals/1'>C-terminal portion of RCL*</scene> in maspin leaded maspin to a dominant nuclear distribution and increased interaction with HDAC1 in multiple cancer cell lines <ref name="numero16">PMID: 24278104</ref>. | ||
| + | |||
<b>*</b> <b><i>Rainbow color</i> schemes orientation:</b> | <b>*</b> <b><i>Rainbow color</i> schemes orientation:</b> | ||
| Line 42: | Line 52: | ||
====Bulge around D and E-helices==== | ====Bulge around D and E-helices==== | ||
| - | Maspin also contains a buried <scene name='91/915218/Saltbridge/1'>salt bridge</scene> between <scene name='91/915218/90_115/3'>Lys90 and Glu115</scene> (where <font color='blue'><b>positive</b></font> and <font color='red'><b>negative</b></font> charged, as well as <span style="color:white;background-color:darkgrey;font-weight:bold;">neutral net charge</span> are highlighted) inside a cavity formed by residues from <scene name='91/915218/D_helix/2'>D-helix</scene>, the B-helix and s2A. This intramolecular interaction causes a prominent <scene name='91/915218/Maspin_bulge/3'>bulge</scene> at the N-terminal end of s1A and reveals a cavity below the D-helix. The salt bridge leads to a bulge around the <b>D-</b> and <scene name='91/915218/E_helix/1'>E-helix</scene> and is, possibly, a relevant cofactor recognition site. It is suggested that this salt bridge region, as in other serpins, may have some relevance at the interaction with a binding partner. Furthermore, the distortion in secondary structure caused by the salt bridge introduces <scene name='91/915218/Lys114/ | + | Maspin also contains a buried <scene name='91/915218/Saltbridge/1'>salt bridge</scene> between <scene name='91/915218/90_115/3'>Lys90 and Glu115</scene> (where <font color='blue'><b>positive</b></font> and <font color='red'><b>negative</b></font> charged, as well as <span style="color:white;background-color:darkgrey;font-weight:bold;">neutral net charge</span> are highlighted) inside a cavity formed by residues from <scene name='91/915218/D_helix/2'>D-helix</scene>, the B-helix and s2A. This intramolecular interaction causes a prominent <scene name='91/915218/Maspin_bulge/3'>bulge</scene> at the N-terminal end of s1A and reveals a cavity below the D-helix. The salt bridge leads to a bulge around the <b>D-</b> and <scene name='91/915218/E_helix/1'>E-helix</scene> and is, possibly, a relevant cofactor recognition site. It is suggested that this salt bridge region, as in other serpins, may have some relevance at the interaction with a binding partner. Furthermore, the distortion in secondary structure caused by the salt bridge introduces <scene name='91/915218/Lys114/2'>Lys114</scene> into the center of a cluster of conserved <font color='blue'><b>positively</b></font> charged waste. Once maspin is able to bind heparin, it is possible that these residues perform as a heparin binding site <ref name="P" />. |
| - | [[Image:Imagem_Ghelice_aberta_vs_fechada1.png|thumb|left|578x325px|<b>Fig. | + | [[Image:Imagem_Ghelice_aberta_vs_fechada1.png|thumb|left|578x325px|<b>Fig. 5</b> - Maspin on its closed (PDB code [[1xqg]]) and opened (PDB code [[1wz9]]) form based on the ideia of the conformation "switch" in wich the rotation of G-helix changes maspin's charge distribuition <ref name="P" />. <font color='blue'><b>Positive</b></font> and <font color='red'><b>negative</b></font> charges can be seen in blue and red respectively, likewise <span style="color:white;background-color:darkgrey;font-weight:bold;">neutral net charge</span> are represented by whyte color. The region comprising the G-helix is <span style="color:black;background-color:yellow;font-weight:bold;">highlighted</span>.]] |
====G-helix==== | ====G-helix==== | ||
Maspin is able to undergo conformational change in and around the <scene name='91/915218/G_helix/2'>G-helix</scene>, presenting two shapes: <b>an <scene name='91/915218/Open_masp_cartoon/3'>opened</scene> and a <scene name='91/915218/Closed_masp_cartoon/3'>closed</scene> form</b>. This is a real putative cofactor binding site and may determine maspin’s function. | Maspin is able to undergo conformational change in and around the <scene name='91/915218/G_helix/2'>G-helix</scene>, presenting two shapes: <b>an <scene name='91/915218/Open_masp_cartoon/3'>opened</scene> and a <scene name='91/915218/Closed_masp_cartoon/3'>closed</scene> form</b>. This is a real putative cofactor binding site and may determine maspin’s function. | ||
| - | Once the region at and around the G-helix has flexibility to undergo a conformational change, as a consequence, its <scene name='91/915218/G_helix_charged/1'>charged residues</scene> (where <font color='blue'><b>positive</b></font> and <font color='red'><b>negative</b></font> charges, as well as <span style="color:white;background-color:darkgrey;font-weight:bold;">neutral net charge</span> are highlighted) are reorganized and the central part of the helix structure becomes negative. The two structures, reflecting maspin in the open and closed conformation, show that rotation of the G-helix alters the local charge distribution (Fig. | + | Once the region at and around the G-helix has flexibility to undergo a conformational change, as a consequence, its <scene name='91/915218/G_helix_charged/1'>charged residues</scene> (where <font color='blue'><b>positive</b></font> and <font color='red'><b>negative</b></font> charges, as well as <span style="color:white;background-color:darkgrey;font-weight:bold;">neutral net charge</span> are highlighted) are reorganized and the central part of the helix structure becomes negative. The two structures, reflecting maspin in the open and closed conformation, show that rotation of the G-helix alters the local charge distribution (Fig. 5), suggesting that this movement represents a conformational “switch” (as we can see on these spacefill representations of <scene name='91/915218/Open_maspin/2'>opened</scene> and <scene name='91/915218/Closed_maspin/2'>closed</scene> forms of maspin), what researchers have implied to be a cofactor binding site under conformational control based on G-helix negative charged patch modulation <ref name="P" />. |
| Line 59: | Line 69: | ||
====Histone Deacetylase 1 (HDAC1)==== | ====Histone Deacetylase 1 (HDAC1)==== | ||
| - | Maspin is able to bind to | + | Maspin is able to bind to [[histone deacetylase]] 1 <b>(HDAC1)</b>, which is up-regulated in many types of cancers and is an important class I nuclear deacetylase <ref name="numero16" />. Either purified or endogenously expressed maspin is bound to and inhibits HDAC1. Maspin/HDAC1 interation in human protate tissues has been examined using GSH-affinity pull-down assay <ref name="numero17">PMID: 16982778</ref>. This interaction and, hence, the inhibition of HDAC1, may allow maspin to control a small set of genes involved in epithelial differentiation <ref name="numero18">PMID: 22737267</ref>. The inhibition of HDAC1 by maspin lead to increased acetylation of HDAC1 target protein Ku70, which in turn, caused an increase in apoptosis <ref name="numero19">PMID: 22076034</ref>. |
Unfortunately, it is not known where the exact binding site of maspin to HDAC1 is until the date of creation of this page (07/20/2022) and more studies are still needed. | Unfortunately, it is not known where the exact binding site of maspin to HDAC1 is until the date of creation of this page (07/20/2022) and more studies are still needed. | ||
Current revision
SerpinB5 (Maspin)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Khalkhali-Ellis Z. Maspin: the new frontier. Clin Cancer Res. 2006 Dec 15;12(24):7279-83. doi: 10.1158/1078-0432.CCR-06-1589. PMID:17189399 doi:http://dx.doi.org/10.1158/1078-0432.CCR-06-1589
- ↑ 2.0 2.1 Banias L, Jung I, Gurzu S. Subcellular expression of maspin – from normal tissue to tumor cells. World J Meta-Anal 2019; 7(4): 142-155. doi: https://dx.doi.org/10.13105/wjma.v7.i4.142
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Law RH, Irving JA, Buckle AM, Ruzyla K, Buzza M, Bashtannyk-Puhalovich TA, Beddoe TC, Nguyen K, Worrall DM, Bottomley SP, Bird PI, Rossjohn J, Whisstock JC. The high resolution crystal structure of the human tumor suppressor maspin reveals a novel conformational switch in the G-helix. J Biol Chem. 2005 Jun 10;280(23):22356-64. Epub 2005 Mar 10. PMID:15760906 doi:http://dx.doi.org/10.1074/jbc.M412043200
- ↑ 4.0 4.1 4.2 4.3 Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI, Whisstock JC. An overview of the serpin superfamily. Genome Biol. 2006;7(5):216. doi: 10.1186/gb-2006-7-5-216. Epub 2006 May 30. PMID:16737556 doi:http://dx.doi.org/10.1186/gb-2006-7-5-216
- ↑ Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000 Oct 19;407(6806):923-6. PMID:11057674 doi:10.1038/35038119
- ↑ Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994 Jan 28;263(5146):526-9. PMID:8290962
- ↑ Goulet B, Kennette W, Ablack A, Postenka CO, Hague MN, Mymryk JS, Tuck AB, Giguere V, Chambers AF, Lewis JD. Nuclear localization of maspin is essential for its inhibition of tumor growth and metastasis. Lab Invest. 2011 Aug;91(8):1181-7. doi: 10.1038/labinvest.2011.66. Epub 2011 Apr , 18. PMID:21502940 doi:http://dx.doi.org/10.1038/labinvest.2011.66
- ↑ 8.0 8.1 Bodenstine TM, Seftor RE, Khalkhali-Ellis Z, Seftor EA, Pemberton PA, Hendrix MJ. Maspin: molecular mechanisms and therapeutic implications. Cancer Metastasis Rev. 2012 Dec;31(3-4):529-51. doi: 10.1007/s10555-012-9361-0. PMID:22752408 doi:http://dx.doi.org/10.1007/s10555-012-9361-0
- ↑ 9.0 9.1 9.2 Yin S, Li X, Meng Y, Finley RL Jr, Sakr W, Yang H, Reddy N, Sheng S. Tumor-suppressive maspin regulates cell response to oxidative stress by direct interaction with glutathione S-transferase. J Biol Chem. 2005 Oct 14;280(41):34985-96. doi: 10.1074/jbc.M503522200. Epub 2005, Jul 26. PMID:16049007 doi:http://dx.doi.org/10.1074/jbc.M503522200
- ↑ Longhi MT, Silva LE, Pereira M, Magalhaes M, Reina J, Vitorino FNL, Gumbiner BM, da Cunha JPC, Cella N. PI3K-AKT, JAK2-STAT3 pathways and cell-cell contact regulate maspin subcellular localization. Cell Commun Signal. 2021 Aug 14;19(1):86. doi: 10.1186/s12964-021-00758-3. PMID:34391444 doi:http://dx.doi.org/10.1186/s12964-021-00758-3
- ↑ Al-Ayyoubi M, Gettins PG, Volz K. Crystal structure of human maspin, a serpin with antitumor properties: reactive center loop of maspin is exposed but constrained. J Biol Chem. 2004 Dec 31;279(53):55540-4. Epub 2004 Oct 22. PMID:15501821 doi:10.1074/jbc.M409957200
- ↑ Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O'Donnell E, Salvesen GS, Travis J, Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001 Sep 7;276(36):33293-6. doi: 10.1074/jbc.R100016200. Epub 2001, Jul 2. PMID:11435447 doi:http://dx.doi.org/10.1074/jbc.R100016200
- ↑ Sheng S, Pemberton PA, Sager R. Production, purification, and characterization of recombinant maspin proteins. J Biol Chem. 1994 Dec 9;269(49):30988-93. PMID:7983035
- ↑ Pemberton PA, Wong DT, Gibson HL, Kiefer MC, Fitzpatrick PA, Sager R, Barr PJ. The tumor suppressor maspin does not undergo the stressed to relaxed transition or inhibit trypsin-like serine proteases. Evidence that maspin is not a protease inhibitory serpin. J Biol Chem. 1995 Jun 30;270(26):15832-7. PMID:7797587
- ↑ Bass R, Fernandez AM, Ellis V. Maspin inhibits cell migration in the absence of protease inhibitory activity. J Biol Chem. 2002 Dec 6;277(49):46845-8. doi: 10.1074/jbc.C200532200. Epub 2002, Oct 15. PMID:12384513 doi:http://dx.doi.org/10.1074/jbc.C200532200
- ↑ 16.0 16.1 Ngamkitidechakul C, Warejcka DJ, Burke JM, O'Brien WJ, Twining SS. Sufficiency of the reactive site loop of maspin for induction of cell-matrix adhesion and inhibition of cell invasion. Conversion of ovalbumin to a maspin-like molecule. J Biol Chem. 2003 Aug 22;278(34):31796-806. doi: 10.1074/jbc.M302408200. Epub, 2003 Jun 10. PMID:12799381 doi:http://dx.doi.org/10.1074/jbc.M302408200
- ↑ 17.0 17.1 17.2 Al-Ayyoubi M, Gettins PG, Volz K. Crystal structure of human maspin, a serpin with antitumor properties: reactive center loop of maspin is exposed but constrained. J Biol Chem. 2004 Dec 31;279(53):55540-4. Epub 2004 Oct 22. PMID:15501821 doi:10.1074/jbc.M409957200
- ↑ 18.0 18.1 Dzinic SH, Kaplun A, Li X, Bernardo M, Meng Y, Dean I, Krass D, Stemmer P, Shin N, Lonardo F, Sheng S. Identification of an intrinsic determinant critical for maspin subcellular localization and function. PLoS One. 2013 Nov 21;8(11):e74502. doi: 10.1371/journal.pone.0074502., eCollection 2013. PMID:24278104 doi:http://dx.doi.org/10.1371/journal.pone.0074502
- ↑ Li X, Yin S, Meng Y, Sakr W, Sheng S. Endogenous inhibition of histone deacetylase 1 by tumor-suppressive maspin. Cancer Res. 2006 Sep 15;66(18):9323-9. doi: 10.1158/0008-5472.CAN-06-1578. PMID:16982778 doi:http://dx.doi.org/10.1158/0008-5472.CAN-06-1578
- ↑ Bernardo MM, Meng Y, Lockett J, Dyson G, Dombkowski A, Kaplun A, Li X, Yin S, Dzinic S, Olive M, Dean I, Krass D, Moin K, Bonfil RD, Cher M, Sakr W, Sheng S. Maspin reprograms the gene expression profile of prostate carcinoma cells for differentiation. Genes Cancer. 2011 Nov;2(11):1009-22. doi: 10.1177/1947601912440170. PMID:22737267 doi:http://dx.doi.org/10.1177/1947601912440170
- ↑ Lee SJ, Jang H, Park C. Maspin increases Ku70 acetylation and Bax-mediated cell death in cancer cells. Int J Mol Med. 2012 Feb;29(2):225-30. doi: 10.3892/ijmm.2011.833. Epub 2011 Nov, 10. PMID:22076034 doi:http://dx.doi.org/10.3892/ijmm.2011.833
- ↑ Singhal SS, Singh SP, Singhal P, Horne D, Singhal J, Awasthi S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol Appl Pharmacol. 2015 Dec 15;289(3):361-70. doi:, 10.1016/j.taap.2015.10.006. Epub 2015 Oct 23. PMID:26476300 doi:http://dx.doi.org/10.1016/j.taap.2015.10.006
- ↑ Nawata S, Shi HY, Sugino N, Zhang M. Evidence of post-translational modification of the tumor suppressor maspin under oxidative stress. Int J Mol Med. 2011 Feb;27(2):249-54. doi: 10.3892/ijmm.2010.572. Epub 2010 Dec, 2. PMID:21132260 doi:http://dx.doi.org/10.3892/ijmm.2010.572