Sandbox Reserved 1792
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 20: | Line 20: | ||
=== Hinge Region=== | === Hinge Region=== | ||
| - | The <scene name='95/952720/Hinge_region_spin/1'>Hinge Region</scene> (purple-blue) connects the Transmembrane Region to the Leucine Rich Domain. Also referred to as the signaling specificity domain the hinge region plays a dual role in both TSH binding and signal transduction. <ref name="Chen">Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]</ref>. The hinge region is made up of two α-helices connected via di-sulfide bonds. | + | The <scene name='95/952720/Hinge_region_spin/1'>Hinge Region</scene> (purple-blue) connects the Transmembrane Region to the Leucine Rich Domain. Also referred to as the signaling specificity domain the hinge region plays a dual role in both TSH binding and signal transduction. <ref name="Chen">Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]</ref>. The hinge region is made up of two α-helices connected via di-sulfide bonds. The disulfide bonds are important for transmitting the signal to the Transmembrane region. The two helices help orient TSH properly for LRRD binding. It is proposed that the <scene name='95/952720/Hinge_tsh_residue/1'>Hinge Region residues</scene> Asp386, Tyr385, and Tyr387 create a negative-charged region on the Helix. This negatively charged region interacts with the positively charged region of TSH created by residue Arg54. These interactions are essential for TSH binding, however, they are not required for the activation of TSHR. This is known because the M22 antibody is able to activate TSHR even though it does not have an Arg54 residue Conformational changes in this hinge region, specifically the orientation of <scene name='95/952720/Hinge_region_residues/2'>Y279 residue</scene>, which is located in the hinge region, are responsible for the bringing TSHR into the active state <ref name="Faust"/> |
== Active vs Inactive State== | == Active vs Inactive State== | ||
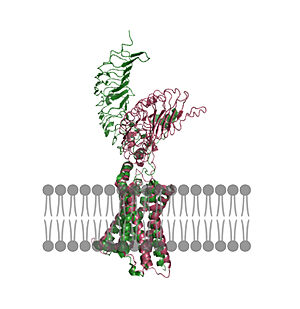
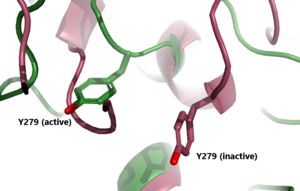
| - | + | In its resting state without TSH bound, TSHR is in the <scene name='95/952720/Inactivetshr/8'>inactive state</scene>, also known as the "down" state because the LRRD is pointing down. When TSH binds to TSHR, steric clashes between TSH and the cell-membrane cause TSHR to take on the <scene name='95/952720/Inactivetshr/6'>active or "up" state</scene> (fig 2). During this transition, the LRRD rotate 55° along an axis perpendicular to the cell membrane. This rotation is initiated by conformational changes within the <scene name='95/952720/Hinge_region_spin/1'>Hinge Region</scene>, specifically at the <scene name='95/952720/Hinge_region_residues/2'>Tyr279 residue</scene>, located in the Hinge Region. Y279 moves 6 Å relative to I486, which is a residue located in the Transmembrane Region nearby Y279<ref name="Faust"/> (Fig 3). | |
| - | + | ||
| + | {| | ||
| + | | [[Image:Untitled (1).jpg|300 px|right|thumb| Figure 2: An overview of the Inactive (pink) vs Active (green) state of TSHR embedded in the plasma membrane. In the inactive state, the LRRD is pointed down. When TSH binds to the LRRD, confirmation changes in the Hinge Region and steric clashes between TSH and the Cell membrane cause TSHR to rotate into the active state. As shown, the LRRD rotates 55° into an upright position <ref name="Faust"/>. PDB: [https://www.rcsb.org/structure/7T9M 7t9m] and [https://www.rcsb.org/structure/7T9I 7t9i].]] | ||
| + | | [[Image:Inactive v active residue.png|300 px|right|thumb| Figure 3: A zoomed in view of the Y279 residue in the Hinge Region of TSHR, showing the 6Å rearrangement of Y279 during the activation of TSHR. Active TSHR is shown in green (PDB: [https://www.rcsb.org/structure/7T9I 7t9i]) and inactive TSHR is shown in pink (PDB: [https://www.rcsb.org/structure/7T9M 7t9m]).]] | ||
| + | |} | ||
== Specific Residues == | == Specific Residues == | ||
Current revision
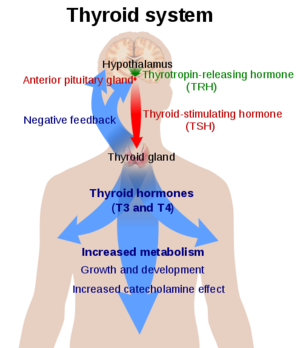
Thyroid Stimulating Hormone Receptor (TSHR)
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 3.2 3.3 3.4 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ 4.0 4.1 4.2 4.3 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ 5.0 5.1 Goel R, Raju R, Maharudraiah J, Sameer Kumar GS, Ghosh K, Kumar A, Lakshmi TP, Sharma J, Sharma R, Balakrishnan L, Pan A, Kandasamy K, Christopher R, Krishna V, Mohan SS, Harsha HC, Mathur PP, Pandey A, Keshava Prasad TS. A Signaling Network of Thyroid-Stimulating Hormone. J Proteomics Bioinform. 2011 Oct 29;4:10.4172/jpb.1000195. PMID:24255551 doi:10.4172/jpb.1000195
- ↑ Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]
Student Contributors
- Alex Kem
- Grace Lane