Sandbox Reserved 1791
From Proteopedia
(Difference between revisions)
| (23 intermediate revisions not shown.) | |||
| Line 4: | Line 4: | ||
== Introduction == | == Introduction == | ||
| - | [[Image:TSH system1.png|300 px|right|thumb| Figure 1: An overview of the Thyroid System. A depiction of signaling cascade from the hypothalamus ending in the release of TSH causing T3 and T4 production and its effects. The mechanism of regulation is also shown by negative feedback from the T3 and T4 hormones. Source: [https://commons.wikimedia.org/wiki/File:Figure_37_04_01.png]]] | + | [[Image:TSH system1.png|300 px|right|thumb| Figure 1: An overview of the Thyroid System. A depiction of signaling cascade from the hypothalamus ending in the release of TSH causing T3 and T4 production and its effects. The mechanism of regulation is also shown by [https://biologydictionary.net/negative-feedback/ negative feedback] from the T3 and T4 hormones. Source: [https://commons.wikimedia.org/wiki/File:Figure_37_04_01.png]]] |
| - | '''Thyroid Stimulating Hormone Receptor (TSHR)''' is a [https://proteopedia.org/wiki/index.php/GPCRs G-Protein Coupled Receptor (GPCR)] found in human thyroid follicles<ref name="Faust"> DOI 10.1038/s41586-022-05159-1</ref>. TSHR is activated by the [https://en.wikipedia.org/wiki/Thyroid-stimulating_hormone Thyroid Stimulating Hormone (TSH)] also known as thyrotropin. Activation of TSHR initiates a signaling pathway for the production of thyroid hormones such as [https://en.wikipedia.org/wiki/Triiodothyronine T<sub>3</sub>] and [https://en.wikipedia.org/wiki/Thyroid_hormones T<sub>4</sub>] | + | '''Thyroid Stimulating Hormone Receptor (TSHR)''' is a [https://proteopedia.org/wiki/index.php/GPCRs G-Protein Coupled Receptor (GPCR)] found in human thyroid follicles<ref name="Faust"> DOI 10.1038/s41586-022-05159-1</ref>. TSHR is activated by the [https://en.wikipedia.org/wiki/Thyroid-stimulating_hormone Thyroid Stimulating Hormone (TSH)] also known as thyrotropin. Activation of TSHR initiates a signaling pathway for the production of thyroid hormones such as [https://en.wikipedia.org/wiki/Triiodothyronine T<sub>3</sub>] and [https://en.wikipedia.org/wiki/Thyroid_hormones T<sub>4</sub>] that are used to regulate the metabolism(Fig 1). |
== Structure== | == Structure== | ||
TSHR forms an active signaling complex with TSH and G<sub>s</sub> proteins. This is called the <scene name=scene name='95/952720/Tsh-tshr-gs_complex/3'>TSH-TSHR-Gs Complex</scene>. TSH contains an α and a β subunit. The α subunit is a shared subunit amongst glycoproteins. The β subunit is unique to TSH. TSH binds to the extracellular domain of TSHR <ref name="Duan"> DOI 10.1038/s41586-022-05173-3</ref>. | TSHR forms an active signaling complex with TSH and G<sub>s</sub> proteins. This is called the <scene name=scene name='95/952720/Tsh-tshr-gs_complex/3'>TSH-TSHR-Gs Complex</scene>. TSH contains an α and a β subunit. The α subunit is a shared subunit amongst glycoproteins. The β subunit is unique to TSH. TSH binds to the extracellular domain of TSHR <ref name="Duan"> DOI 10.1038/s41586-022-05173-3</ref>. | ||
<scene name=scene name='95/952720/Structure_overview_spins/3'> | <scene name=scene name='95/952720/Structure_overview_spins/3'> | ||
| - | TSHR has 3 main domains</scene>: Leucine Rich Region Domain (coral), the hinge region (blue-purple), and the transmembrane region(rainbow). The leucine rich region domain is the extracellular TSH ligand domain. The hinge connects the Leucine Rich Repeat Domain and the Transmembrane Region. It provides flexibility for the switch between the active and inactive state of TSHR. The transmembrane region is located within the plasma membrane. Its function | + | TSHR has 3 main domains</scene>: Leucine Rich Region Domain (coral), the hinge region (blue-purple), and the transmembrane region(rainbow). The leucine-rich region domain is the extracellular TSH ligand domain. The hinge connects the Leucine Rich Repeat Domain and the Transmembrane Region. It provides flexibility for the switch between the active and inactive state of TSHR. The transmembrane region is located within the plasma membrane. Its function transmits the extracellular signal across the membrane to the intracellular [https://en.wikipedia.org/wiki/G_protein G-proteins] bound to the N-terminus of the transmembrane region<ref name="Duan"/>. Activated G-proteins then signal a robust intracellular signaling cascade. |
=== Leucine Rich Domain=== | === Leucine Rich Domain=== | ||
| - | The <scene name='95/952719/Lrrd/1'>Leucine Rich Repeat Domain (LRRD)</scene> is the extracellular ligand binding region of TSHR. Connects to the Hinge Region at its C-terminus. It is made up of polar residues in a concave parallel β-sheet composed of leucine-rich repeats where TSH binds and is called the <scene name='95/952719/Binding_pocket/7'>binding pocket</scene><ref name="Duan"> DOI 10.1038/s41586-022-05173-3</ref>. The LRRD concave surface is where the TSH antibody antagonist, K1, binds as well as the agonist M22. Two specific LYS residues interact with the substrate to result in a structural change of the molecule. | + | The <scene name='95/952719/Lrrd/1'>Leucine Rich Repeat Domain (LRRD)</scene> is the extracellular ligand binding region of TSHR. Connects to the Hinge Region at its C-terminus. It is made up of polar residues in a concave parallel β-sheet composed of leucine-rich repeats where TSH binds and is called the <scene name='95/952719/Binding_pocket/7'>binding pocket</scene><ref name="Duan"> DOI 10.1038/s41586-022-05173-3</ref>. The LRRD concave surface is where the TSH antibody antagonist, K1, binds as well as the agonist M22. Two specific LYS residues interact with the substrate to result in a structural change of the molecule. [https://pubmed.ncbi.nlm.nih.gov/35536965/ N glycans] attached to asparagine residues play a large role in the binding of TSH. Negative charge on these N glycans contributes to the polarity of the binding pocket which mediates the binding efficiency of TSH. TSHR can be glycosylated at residues 77, 99, 113, 177, 198, and 302<ref name="Fokina">Fokina, E.F., Shpakov, A.O. Thyroid-Stimulating Hormone Receptor: the Role in the Development of Thyroid Pathology and Its Correction. J Evol Biochem Phys 58, 1439–1454 (2022). [DOI:10.1134/S0022093022050143 https://doi.org/10.1134/S0022093022050143]</ref>. Four of the five N glycan sites must be glycosylated for TSHR to be in the active form<ref name="Fokina">Fokina, E.F., Shpakov, A.O. Thyroid-Stimulating Hormone Receptor: the Role in the Development of Thyroid Pathology and Its Correction. J Evol Biochem Phys 58, 1439–1454 (2022). [DOI:10.1134/S0022093022050143 https://doi.org/10.1134/S0022093022050143]</ref>. |
=== Hinge Region=== | === Hinge Region=== | ||
| - | The <scene name='95/952719/Hinge_region_spin/3'>Higne Region</scene>(purple-blue) connects the Transmembrane Region to the Leucine Rich Domain. Also referred to as the signaling specificity domain the hinge region plays a dual role in both TSH binding and signal transduction. <ref name="Chen">Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]</ref>. The hinge region is made up of two α-helices connected via disulfide bonds. The disulfide bonds are important for transmitting the signal to the Transmembrane region. The two helices help orient TSH properly for LRRD binding. It is proposed that the <scene name='95/952720/Hinge_tsh_residue/1'>Hinge Region residues</scene> Asp386, Tyr385, and Tyr387 create a negative-charged region on the Helix. This negatively charged region interacts with the positively charged region of TSH created by residue Arg54. These interactions are essential for TSH binding, however, they are not required for the activation of TSHR. This is known because the M22 antibody is able to activate TSHR even though it does not have an Arg54 residue Conformational changes in this hinge region, specifically the orientation of <scene name='95/ | + | The <scene name='95/952719/Hinge_region_spin/3'>Higne Region</scene>(purple-blue) connects the Transmembrane Region to the Leucine Rich Domain. Also referred to as the signaling specificity domain the hinge region plays a dual role in both TSH binding and signal transduction. <ref name="Chen">Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]</ref>. The hinge region is made up of two α-helices connected via disulfide bonds. The disulfide bonds are important for transmitting the signal to the Transmembrane region. The two helices help orient TSH properly for LRRD binding. It is proposed that the <scene name='95/952720/Hinge_tsh_residue/1'>Hinge Region residues</scene> Asp386, Tyr385, and Tyr387 create a negative-charged region on the Helix. This negatively charged region interacts with the positively charged region of TSH created by residue Arg54. These interactions are essential for TSH binding, however, they are not required for the activation of TSHR. This is known because the M22 antibody is able to activate TSHR even though it does not have an Arg54 residue Conformational changes in this hinge region, specifically the orientation of <scene name='95/952719/Hinge_region_residues/4'>Y279</scene>, which is located in the hinge region, are responsible for the bringing TSHR into the active state <ref name="Faust"/>. |
=== Transmembrane Region=== | === Transmembrane Region=== | ||
| - | <scene name='95/952720/Transmembrane_region_spin/5'>The Transmembrane Region</scene> (<scene name='95/952720/Transmembrane_region_top-view/2'>top-view</scene>) is embedded within the cell membrane, like other G-protein receptors, it is composed of a 7-pass helix <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref>. The transmembrane region is surrounded by a "belt" of <scene name='95/952719/Tmd_cholesterol_spin/ | + | <scene name='95/952720/Transmembrane_region_spin/5'>The Transmembrane Region</scene> (<scene name='95/952720/Transmembrane_region_top-view/2'>top-view</scene>) is embedded within the cell membrane, like other G-protein receptors, it is composed of a 7-pass helix <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref>. The transmembrane region is surrounded by a "belt" of <scene name='95/952719/Tmd_cholesterol_spin/4'>15 cholesterols</scene>. When cholesterol binding sites are mutated, TSHR activity decreases. These cholesterols are likely important for TSHR function <ref name="Duan"/>. Additionally, at the N-terminus, the transmembrane region binds to the <scene name='95/952719/Tsh-tshr-gs_complex/3'>G-Proteins</scene>, which are located intracellularly <ref name="GOEL"/>. The G-proteins are made up of three subunits: α, β, and γ. Nb35 a fourth subunit that stabilizes the structure by binding between the Gα and Gβ interface<ref name="Maeda"> DOI: 10.1038/s41467-018-06002-w</ref>. When TSHR is activated, it causes the Gα subunit to dissociate from the Gβγ subunits. The Gα subunit is responsible for activating [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylyl cyclase], [https://en.wikipedia.org/wiki/Phospholipase_C phospholipase C], and [https://en.wikipedia.org/wiki/Ion_channel ion channels]. This sets off the robust intracellular signaling cascade<ref name="GOEL"> PMID:24255551</ref>. |
== Antibodies == | == Antibodies == | ||
| - | [https://my.clevelandclinic.org/health/body/22971-antibodies Antibodies] are | + | [https://my.clevelandclinic.org/health/body/22971-antibodies Antibodies] are important for the thyroid response to regulate the metabolism. These proteins are made from an immune system response to get rid of unwanted antigens in the body. [https://www.btf-thyroid.org/thyroid-antibodies-explained Thyroid antibodies] are made when the body attacks the thyroid tissues. These antibodies are made to mimic [https://my.clevelandclinic.org/health/articles/23524-thyroid-stimulating-hormone-tsh-levels TSH (Thyroid Stimulating Hormone)]. TSH is released from the pituitary gland to bind to TSHR and stimulate the thyroid to make T3 and T4 hormones to regulate the metabolism. These thyroid antibodies bind to the concave surface of the LRRD. |
| - | === M22 === | + | === M22 and K1 === |
| - | M22 is an activating antibody for [https://en.wikipedia.org/wiki/Thyrotropin_receptor TSHR]. This antibody mimics [https://my.clevelandclinic.org/health/articles/23524-thyroid-stimulating-hormone-tsh-levels TSH] to activate the thyroid gland to produce [https://www.healthyandnaturalworld.com/t3-t4-thyroid-hormones/ T3 and T4 hormones]. M22 makes a stronger interaction with TSHR than TSH does due to a larger number of hydrogen bonds and salt | + | M22 is an activating antibody for [https://en.wikipedia.org/wiki/Thyrotropin_receptor TSHR]. This antibody mimics [https://my.clevelandclinic.org/health/articles/23524-thyroid-stimulating-hormone-tsh-levels TSH] to activate the thyroid gland to produce [https://www.healthyandnaturalworld.com/t3-t4-thyroid-hormones/ T3 and T4 hormones]. M22 makes a stronger interaction with TSHR than TSH does due to a larger number of hydrogen bonds (14 H bonds for M22 and 3 for TSH) and salt bridge interactions with the concave surface of the LRRD<ref name="M22"> DOI 10.1677/JME-08-0152</ref>. This interaction is key for understanding why M22 activates TSHR and does not release TSHR to go into the inactive state even when T3 and T4 levels are high<ref name="M22"> DOI 10.1677/JME-08-0152</ref>. |
| - | + | [https://www.creativebiolabs.net/Anti-TSHR-Recombinant-Antibody-clone-K1-70-24911.htm K1] is an inhibitory antibody. This antibody mimics [https://my.clevelandclinic.org/health/articles/23524-thyroid-stimulating-hormone-tsh-levels TSH] and binds to TSHR with a high affinity which prevents the receptor binding to TSH. K1 puts TSHR in the inactive conformation<ref name="K1"> Furmaniak J, Sanders J, Sanders P, Li Y, Rees Smith B. TSH receptor specific monoclonal autoantibody K1-70TM targeting of the TSH receptor in subjects with Graves' disease and Graves' orbitopathy-Results from a phase I clinical trial. Clin Endocrinol (Oxf). 2022 Jun;96(6):878-887. doi: 10.1111/cen.14681. Epub 2022 Feb 6. PMID: 35088429; PMCID: PMC9305464. [DOI 10.1111/cen.14681]</ref>. It is effective at lowering T3 and T4 levels while increasing TSH concentration. | |
| - | [https://www.creativebiolabs.net/Anti-TSHR-Recombinant-Antibody-clone-K1-70-24911.htm K1] is an inhibitory antibody. This antibody mimics | + | |
== Active vs. Inactive State == | == Active vs. Inactive State == | ||
| - | + | ||
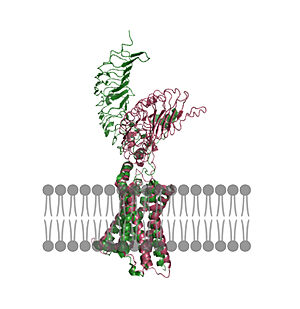
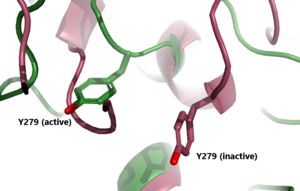
| - | + | In its resting state without TSH bound, TSHR is in the <scene name='95/952720/Inactivetshr/8'>inactive state</scene>, also known as the "down" state because the LRRD is pointing down. When TSH binds to TSHR, steric clashes between TSH and the cell-membrane cause TSHR to take on the <scene name='95/952720/Inactivetshr/6'>active or "up" state</scene> (Fig 2). During this transition, the LRRD rotate 55° along an axis perpendicular to the cell membrane. This rotation is initiated by conformational changes within the <scene name='95/952719/Hinge_region_spin/3'>Higne Region</scene>, specifically at the <scene name='95/952720/Hinge_region_residues/3'>Tyr279 residue</scene>, located in the Hinge Region. Y279 moves 6 Å relative to I486, which is a residue located in the Transmembrane Region nearby Y279<ref name="Faust"/> (Fig 3). | |
| - | In its resting state without TSH, TSHR is in the <scene name='95/952720/Inactivetshr/ | + | The active form is favored when <scene name='95/952719/Active_form/6'>TSHR is bound to TSH</scene>. The structure can be seen as straight. The same straight conformation is observed when TSHR is bound with M22. The inactive form is found when <scene name='95/952719/Inactive_form/7'>TSHR is bound with K1</scene> of TSHR is found when bound with K1. The overall structure of the molecule is bent when K1 is bound. |
{| | {| | ||
| - | | [[Image:Untitled (1).jpg|300 px|right|thumb| Figure 2: An overview of the Inactive (pink) vs Active (green) state of TSHR embedded in the plasma membrane. In the inactive state, the LRRD is pointed down. When TSH binds to the LRRD, | + | | [[Image:Untitled (1).jpg|300 px|right|thumb| Figure 2: An overview of the Inactive (pink) vs Active (green) state of TSHR embedded in the plasma membrane. In the inactive state, the LRRD is pointed down. When TSH binds to the LRRD, conformation changes in the Hinge Region and steric clashes between TSH and the Cell membrane cause TSHR to rotate into the active state. As shown, the LRRD rotates 55° into an upright position <ref name="Faust"/>. PDB: [https://www.rcsb.org/structure/7T9M 7t9m] and [https://www.rcsb.org/structure/7T9I 7t9i].]] |
| [[Image:Inactive v active residue.png|300 px|right|thumb| Figure 3: A zoomed in view of the Y279 residue in the Hinge Region of TSHR, showing the 6Å rearrangement of Y279 during the activation of TSHR. Active TSHR is shown in green (PDB: [https://www.rcsb.org/structure/7T9I 7t9i]) and inactive TSHR is shown in pink (PDB: [https://www.rcsb.org/structure/7T9M 7t9m]).]] | | [[Image:Inactive v active residue.png|300 px|right|thumb| Figure 3: A zoomed in view of the Y279 residue in the Hinge Region of TSHR, showing the 6Å rearrangement of Y279 during the activation of TSHR. Active TSHR is shown in green (PDB: [https://www.rcsb.org/structure/7T9I 7t9i]) and inactive TSHR is shown in pink (PDB: [https://www.rcsb.org/structure/7T9M 7t9m]).]] | ||
|} | |} | ||
== Specific Residues and Interactions== | == Specific Residues and Interactions== | ||
| - | + | On the concave surface of the LRRD two <scene name='95/952719/Specific_residues/6'>lysine residues</scene> are the main contributors to the binding of the antibodies. The concave structure of the binding pocket allows a <scene name='95/952719/Lock_and_key/8'>tight interaction</scene> with antibodies. Specifically, <scene name='95/952719/K---e_interaction/8'>LYS 58</scene> interacts with Glu 118 and <scene name='95/952719/K---d_interaction/9'>LYS 209</scene> interacts with Asp 111 on K1 and M22 antibodies to make a [https://www.nature.com/articles/s41598-018-31935-z salt bridge interaction]. The interaction is not close enough to make a hydrogen bond. Instead, the interaction between the Lys residues with the Asp or Glu residues is a [https://www.nature.com/articles/s41598-018-31935-z salt bridge interaction] because the distance between the residues is greater than 2Å. When in the inactive form, LYS 209 interaction is absent but LYS 58 interaction with Glu 118 is present. These salt bridge interactions are highly specific as mutation of Lys209 to Arg induced a different interaction. This new interaction widens the selectivity of TSHR, allowing it to bind to other hormones<ref name="Guillaume">Smits G, Govaerts C, Nubourgh I, Pardo L, Vassart G, Costagliola S. Lysine 183 and glutamic acid 157 of the TSH receptor: two interacting residues with a key role in determining specificity toward TSH and human CG. Mol Endocrinol. 2002 Apr;16(4):722-35. doi: 10.1210/mend.16.4.0815. PMID: 11923469. [DOI: 10.1210/mend.16.4.0815 https://pubmed.ncbi.nlm.nih.gov/11923469/]</ref>. | |
== Biological Relevance == | == Biological Relevance == | ||
[[Image:T3t4levels.jpeg|400 px|right|thumb|Figure 4: T3 and T4 role in TSH concentration: Highlighting the problem when under or overactive on the metabolism. When an antibody is bound to TSHR and cannot respond to the negative feedback look the metabolism experiences a shift outside of equilibrium resulting in a wide array of side effects. [https://commons.wikimedia.org/wiki/File:1813_A_Classic_Negative_Feedback_Loop.jpg]]] | [[Image:T3t4levels.jpeg|400 px|right|thumb|Figure 4: T3 and T4 role in TSH concentration: Highlighting the problem when under or overactive on the metabolism. When an antibody is bound to TSHR and cannot respond to the negative feedback look the metabolism experiences a shift outside of equilibrium resulting in a wide array of side effects. [https://commons.wikimedia.org/wiki/File:1813_A_Classic_Negative_Feedback_Loop.jpg]]] | ||
| - | The thyroid plays an essential role in the body's metabolism | + | The thyroid plays an essential role in the body's metabolism functions, including heart rate, digestion, and temperature regulation. When TSH is bound to TSHR, a signal is sent to the [https://my.clevelandclinic.org/health/body/23188-thyroid thyroid] to produce the [https://my.clevelandclinic.org/health/articles/22391-thyroid-hormone thyroid hormones], [https://en.wikipedia.org/wiki/Triiodothyronine T<sub>3</sub>] and [https://en.wikipedia.org/wiki/Thyroid_hormones T<sub>4</sub>]. |
| + | T4 is an inactive form until converted into T3. T3 and T4 are transmitted through the body to increase or decrease your metabolism. T3 and T4 then use a feedback mechanism to regulate the release of TSH by the [https://my.clevelandclinic.org/health/body/21459-pituitary-gland pituitary gland](Fig.4). High T3 and T4 levels inhibit TSH production. Whereas low levels permit TSH binding to TSHR. | ||
=== Hyperthyroidism === | === Hyperthyroidism === | ||
| - | [https://www.mayoclinic.org/diseases-conditions/hyperthyroidism/symptoms-causes/syc-20373659 Hyperthyroidism] is when the thyroid is overactive which leads to upregulation of the metabolism. This overactive state can be initiated by antibodies like M22. | + | [https://www.mayoclinic.org/diseases-conditions/hyperthyroidism/symptoms-causes/syc-20373659 Hyperthyroidism] is when the thyroid is overactive which leads to upregulation of the metabolism. This overactive state can be initiated by antibodies like M22. M22 antibody maintains TSHR in the active conformation. In this conformation, the thyroid continues to make T3 and T4, overstimulating the metabolism. Symptoms of hyperthyroidism include fast or irregular heartbeats, tiredness, increased hunger, sleep problems, enlarged thyroid gland, and sensitivity to heat. [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Grave's Disease] is the most common cause of hyperthyroidism. This is an autoimmune disorder that causes your body to attack the thyroid gland<ref name="Luca">Chiovato L, Magri F, Carlé A. Hypothyroidism in Context: Where We've Been and Where We're Going. Adv Ther. 2019 Sep;36(Suppl 2):47-58. doi: 10.1007/s12325-019-01080-8. Epub 2019 Sep 4. PMID: 31485975; PMCID: PMC6822815. [DOI: 10.1007/s12325-019-01080-8 https://pubmed.ncbi.nlm.nih.gov/31485975/]</ref>. When someone has Hypothyroidism the body produces M22 that leads to overactive metabolism functions. |
=== Hypothyroidism === | === Hypothyroidism === | ||
| - | [https://www.mayoclinic.org/diseases-conditions/hypothyroidism/symptoms-causes/syc-20350284#:~:text=Hypothyroidism%20happens%20when%20the%20thyroid,symptoms%20in%20its%20early%20stages Hypothyroidism] is when the thyroid is underactive. | + | [https://www.mayoclinic.org/diseases-conditions/hypothyroidism/symptoms-causes/syc-20350284#:~:text=Hypothyroidism%20happens%20when%20the%20thyroid,symptoms%20in%20its%20early%20stages Hypothyroidism] is when the thyroid is underactive leading to a decrease in metabolism function. K1 antibody binds to TSHR to promote the inactive confirmation that is conserved despite the presence of TSH. This does not allow for the signaling of the T3 and T4 hormones to upregulate the metabolism. The symptoms of this include slow or irregular heartbeats, tiredness, muscle aches, memory problems, [https://my.clevelandclinic.org/health/diseases/15367-adult-jaundice jaundice], and sensitivity to cold. [https://www.mayoclinic.org/diseases-conditions/hashimotos-disease/symptoms-causes/syc-20351855 Hasimoto's disease] is an example of hypothyroidism. This is an autoimmune disorder that causes your body to attack the healthy cells of the thyroid. Specifically causing the death of the cells that produce the thyroid hormones. When the thyroid fails to produce its hormones it activates TSH production through a [https://biologydictionary.net/negative-feedback/ negative feedback mechanism]<ref name="Luca">. When someone has Hashimoto's disease the body produces the K1 antibody it does not respond to the negative feedback system and the body cannot regulate the metabolism. |
== Student Contributions == | == Student Contributions == | ||
*Alex Kem | *Alex Kem | ||
Current revision
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Thyroid Stimulating Hormone Receptor (TSHR)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ 2.0 2.1 2.2 2.3 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ 3.0 3.1 Fokina, E.F., Shpakov, A.O. Thyroid-Stimulating Hormone Receptor: the Role in the Development of Thyroid Pathology and Its Correction. J Evol Biochem Phys 58, 1439–1454 (2022). [DOI:10.1134/S0022093022050143 https://doi.org/10.1134/S0022093022050143]
- ↑ Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]
- ↑ 5.0 5.1 Goel R, Raju R, Maharudraiah J, Sameer Kumar GS, Ghosh K, Kumar A, Lakshmi TP, Sharma J, Sharma R, Balakrishnan L, Pan A, Kandasamy K, Christopher R, Krishna V, Mohan SS, Harsha HC, Mathur PP, Pandey A, Keshava Prasad TS. A Signaling Network of Thyroid-Stimulating Hormone. J Proteomics Bioinform. 2011 Oct 29;4:10.4172/jpb.1000195. PMID:24255551 doi:10.4172/jpb.1000195
- ↑ Maeda S, Koehl A, Matile H, Hu H, Hilger D, Schertler GFX, Manglik A, Skiniotis G, Dawson RJP, Kobilka BK. Development of an antibody fragment that stabilizes GPCR/G-protein complexes. Nat Commun. 2018 Sep 13;9(1):3712. doi: 10.1038/s41467-018-06002-w. PMID:30213947 doi:http://dx.doi.org/10.1038/s41467-018-06002-w
- ↑ 7.0 7.1 Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152
- ↑ Furmaniak J, Sanders J, Sanders P, Li Y, Rees Smith B. TSH receptor specific monoclonal autoantibody K1-70TM targeting of the TSH receptor in subjects with Graves' disease and Graves' orbitopathy-Results from a phase I clinical trial. Clin Endocrinol (Oxf). 2022 Jun;96(6):878-887. doi: 10.1111/cen.14681. Epub 2022 Feb 6. PMID: 35088429; PMCID: PMC9305464. [DOI 10.1111/cen.14681]
- ↑ Smits G, Govaerts C, Nubourgh I, Pardo L, Vassart G, Costagliola S. Lysine 183 and glutamic acid 157 of the TSH receptor: two interacting residues with a key role in determining specificity toward TSH and human CG. Mol Endocrinol. 2002 Apr;16(4):722-35. doi: 10.1210/mend.16.4.0815. PMID: 11923469. [DOI: 10.1210/mend.16.4.0815 https://pubmed.ncbi.nlm.nih.gov/11923469/]
- ↑ 10.0 10.1 Chiovato L, Magri F, Carlé A. Hypothyroidism in Context: Where We've Been and Where We're Going. Adv Ther. 2019 Sep;36(Suppl 2):47-58. doi: 10.1007/s12325-019-01080-8. Epub 2019 Sep 4. PMID: 31485975; PMCID: PMC6822815. [DOI: 10.1007/s12325-019-01080-8 https://pubmed.ncbi.nlm.nih.gov/31485975/]
![Figure 1: An overview of the Thyroid System. A depiction of signaling cascade from the hypothalamus ending in the release of TSH causing T3 and T4 production and its effects. The mechanism of regulation is also shown by negative feedback from the T3 and T4 hormones. Source: [1]](/wiki/images/thumb/1/1d/TSH_system1.png/300px-TSH_system1.png)
![Figure 4: T3 and T4 role in TSH concentration: Highlighting the problem when under or overactive on the metabolism. When an antibody is bound to TSHR and cannot respond to the negative feedback look the metabolism experiences a shift outside of equilibrium resulting in a wide array of side effects. [2]](/wiki/images/thumb/5/5f/T3t4levels.jpeg/400px-T3t4levels.jpeg)
