User:João Pedro de Carvalho Pereira/Sandbox 1
From Proteopedia
< User:João Pedro de Carvalho Pereira(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
== Cystathionine gamma-lyase (''Homo sapiens'') == | == Cystathionine gamma-lyase (''Homo sapiens'') == | ||
| - | <StructureSection load='2nmp' size='340' side='right' caption='Homo sapiens cystathionine gamma-lyase (PDB code [[2nmp]])' scene=''> | + | <StructureSection load='2nmp' size='340' side='right' caption='Homo sapiens cystathionine gamma-lyase (PDB code [[2nmp]]). Each monomer is in a different color.' scene=''> |
Cystathionine gamma-lyase (CSE) is a member of the gamma family of PLP-dependent enzymes (which bind to pyridoxal-5'-phosphate for reaction catalysis), along with other enzymes such as cystathionine gamma-synthase, [[Cystathionine beta-lyase]], and [[Methionine gamma-lyase]]. Similar to other enzymes in this family, CSE exhibits a tetrameric structure with D2 symmetry.<br> | Cystathionine gamma-lyase (CSE) is a member of the gamma family of PLP-dependent enzymes (which bind to pyridoxal-5'-phosphate for reaction catalysis), along with other enzymes such as cystathionine gamma-synthase, [[Cystathionine beta-lyase]], and [[Methionine gamma-lyase]]. Similar to other enzymes in this family, CSE exhibits a tetrameric structure with D2 symmetry.<br> | ||
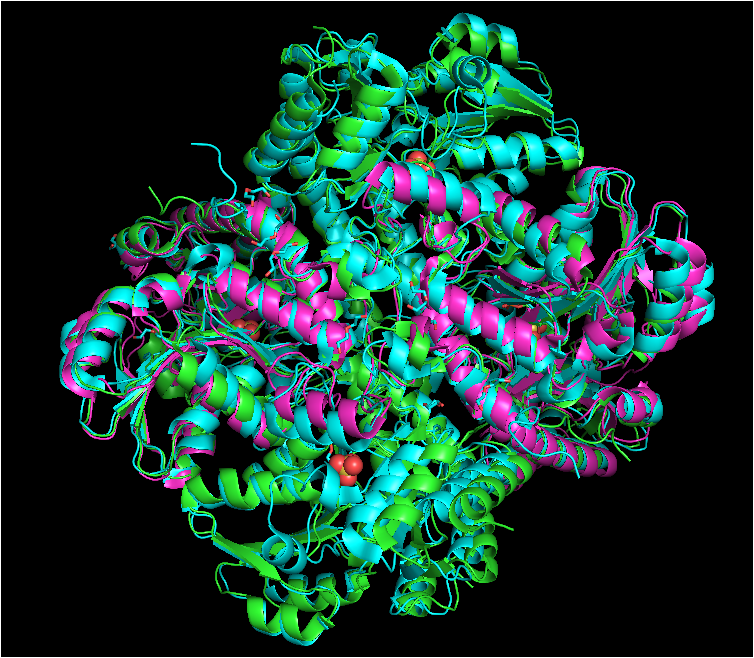
[[Image:Alinhamento proteínas.png|800px|center|thumb| Structure of cystathionine gamma-lyase, [[cystathionine beta-lyase]], and [[methionine gamma-lyase]] proteins aligned. Cystathionine gamma-lyase is shown in green, cystathionine beta-lyase in purple, and methionine gamma-lyase in blue. Structures aligned vy WinCoot and image generated by PyMOL.]] | [[Image:Alinhamento proteínas.png|800px|center|thumb| Structure of cystathionine gamma-lyase, [[cystathionine beta-lyase]], and [[methionine gamma-lyase]] proteins aligned. Cystathionine gamma-lyase is shown in green, cystathionine beta-lyase in purple, and methionine gamma-lyase in blue. Structures aligned vy WinCoot and image generated by PyMOL.]] | ||
| Line 8: | Line 8: | ||
Cystathionine gamma-lyase serves two primary functions in ''Homo sapiens'': one is associated with the conversion of L,L-cystathionine into L-cysteine, while the other is linked to the synthesis of H<sub>2</sub>S (hydrogen sulfide), a signaling molecule implicated in neurological and vascular processes <ref name="um">PMID:19261609</ref>.<br> | Cystathionine gamma-lyase serves two primary functions in ''Homo sapiens'': one is associated with the conversion of L,L-cystathionine into L-cysteine, while the other is linked to the synthesis of H<sub>2</sub>S (hydrogen sulfide), a signaling molecule implicated in neurological and vascular processes <ref name="um">PMID:19261609</ref>.<br> | ||
<br> | <br> | ||
| - | CSE operates in conjunction with cystathionine beta-synthase (CBS) to facilitate the reverse transsulfuration required for the metabolic interconversion of sulfur-containing amino acids, such as cysteine. Remarkably, reverse transsulfuration is a process exclusive to fungi and mammals. In this intricate process, CBS enzymatically catalyzes the formation of cystathionine from the precursor molecules, homocysteine and serine. Subsequently, CSE mediates the conversion of the synthesized cystathionine into cysteine, alpha-ketobutyrate, and ammonia <ref name="dois">PMID:12715888</ref>.<br> | + | CSE operates in conjunction with [[cystathionine beta-synthase]] (CBS) to facilitate the reverse transsulfuration required for the metabolic interconversion of sulfur-containing amino acids, such as cysteine. Remarkably, reverse transsulfuration is a process exclusive to fungi and mammals. In this intricate process, CBS enzymatically catalyzes the formation of cystathionine from the precursor molecules, homocysteine and serine. Subsequently, CSE mediates the conversion of the synthesized cystathionine into cysteine, alpha-ketobutyrate, and ammonia <ref name="dois">PMID:12715888</ref>.<br> |
<br> | <br> | ||
| - | CSE also assumes the responsibility for the synthesis of H<sub>2</sub>S in mammals. This production holds significant importance due to the pivotal role of this compound as a gaseous messenger or gasotransmitter, intricately linked to the functions of the nervous and vascular systems, as well as inflammation. Specifically, CSE is associated with the production of this gasotransmitter in extraneural contexts, whereas CBS fulfills this role within the central nervous system <ref name ="x">PMID:19019829</ref>. The enzyme facilitates the formation of | + | CSE also assumes the responsibility for the synthesis of H<sub>2</sub>S in mammals. This production holds significant importance due to the pivotal role of this compound as a gaseous messenger or gasotransmitter, intricately linked to the functions of the nervous and vascular systems, as well as inflammation. Specifically, CSE is associated with the production of this gasotransmitter in extraneural contexts, whereas CBS fulfills this role within the central nervous system <ref name ="x">PMID:19019829</ref>. The enzyme facilitates the formation of H<sub>2</sub>S through a series of reactions classified into two main categories: cysteine-dependent beta reactions and homocysteine-dependent gamma reactions <ref name="um"/>. |
== Disease == | == Disease == | ||
| Line 20: | Line 20: | ||
The cystathionine gamma-lyase enzymes from multiple organisms form an evolutionarily conserved group of PLP-dependent enzymes, specifically categorized within the cystathionine synthase-like enzyme family. The data exhibits a substantial level of sequence homology among these CSE enzymes at the genomic level. Furthermore, a predominant structural similarity is observed between the active site region of the CSE enzymes and other PLP-dependent enzymes<ref name ="x"/>.<br> | The cystathionine gamma-lyase enzymes from multiple organisms form an evolutionarily conserved group of PLP-dependent enzymes, specifically categorized within the cystathionine synthase-like enzyme family. The data exhibits a substantial level of sequence homology among these CSE enzymes at the genomic level. Furthermore, a predominant structural similarity is observed between the active site region of the CSE enzymes and other PLP-dependent enzymes<ref name ="x"/>.<br> | ||
<br> | <br> | ||
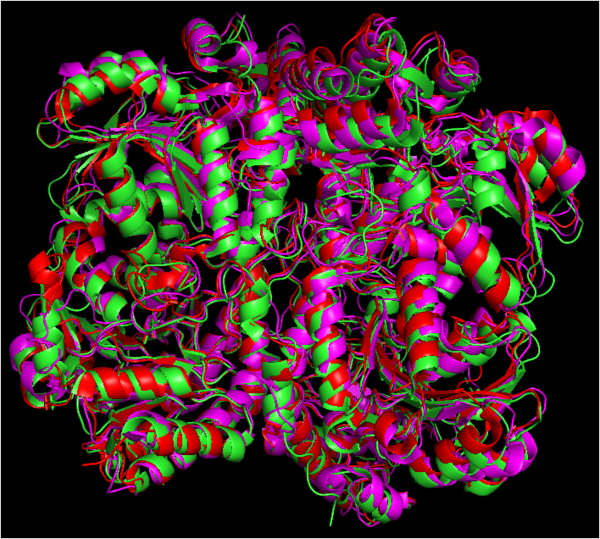
| - | [[Image:Alinhamento gui.png|600px|center|thumb| Alignment of the amino acid sequences of cystathionine gamma-lyase protein from Homo sapiens, Mus musculus, Pseudomonas aeruginosa, and Saccharomyces cerevisiae, showing high similarity across different lineages. Alignment by COBALT NCBI tool.]] | + | [[Image:Alinhamento gui.png|600px|center|thumb| Alignment of the amino acid sequences of cystathionine gamma-lyase protein from Homo sapiens, Mus musculus, Pseudomonas aeruginosa ([[7ba4]]), and Saccharomyces cerevisiae ([[1n8p]]), showing high similarity across different lineages. Alignment by COBALT NCBI tool.]]<br> |
| + | [[Image:Alinhamento cistationina.png|600px|center|thumb| Alignment of the structures of cystathionine gamma-lyase protein from Homo sapiens (green), Pseudomonas aeruginosa (red) and Saccharomyces cerevisiae (purple). Structures aligned vy WinCoot and image generated by PyMOL.]] | ||
== Structure == | == Structure == | ||
Current revision
Cystathionine gamma-lyase (Homo sapiens)
| |||||||||||
References
- ↑ 1.0 1.1 Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009 Apr 24;284(17):11601-12. doi: 10.1074/jbc.M808026200. Epub 2009, Mar 4. PMID:19261609 doi:10.1074/jbc.M808026200
- ↑ Messerschmidt A, Worbs M, Steegborn C, Wahl MC, Huber R, Laber B, Clausen T. Determinants of enzymatic specificity in the Cys-Met-metabolism PLP-dependent enzymes family: crystal structure of cystathionine gamma-lyase from yeast and intrafamiliar structure comparison. Biol Chem. 2003 Mar;384(3):373-86. PMID:12715888
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Sun Q, Collins R, Huang S, Holmberg-Schiavone L, Anand GS, Tan CH, van-den-Berg S, Deng LW, Moore PK, Karlberg T, Sivaraman J. Structural basis for the inhibition mechanism of human cystathionine gamma-lyase, an enzyme responsible for the production of H(2)S. J Biol Chem. 2009 Jan 30;284(5):3076-85. Epub 2008 Nov 19. PMID:19019829 doi:http://dx.doi.org/10.1074/jbc.M805459200
- ↑ 4.0 4.1 Adaikan PG, Karim SM. Effects of PGA and PGB compounds on gastrointestinal tract smooth muscle from man and laboratory animals. Prostaglandins. 1976 Jan;11(1):15-22. PMID:1257494 doi:10.1016/0090-6980(76)90168-4
- ↑ Scott CR, Dassell SW, Clark SH, Chiang-Teng C, Swedberg KR. Cystathioninemia: a benign genetic condition. J Pediatr. 1970 Apr;76(4):571-7. PMID:5420794 doi:10.1016/s0022-3476(70)80407-3
- ↑ Wang J, Hegele RA. Genomic basis of cystathioninuria (MIM 219500) revealed by multiple mutations in cystathionine gamma-lyase (CTH). Hum Genet. 2003 Apr;112(4):404-8. Epub 2003 Feb 6. PMID:12574942 doi:10.1007/s00439-003-0906-8