User:Mathias Vander Eide/Sandbox 1
From Proteopedia
< User:Mathias Vander Eide(Difference between revisions)
| (28 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
=Human Amylin Receptor with Associated RAMP1= | =Human Amylin Receptor with Associated RAMP1= | ||
| - | <StructureSection load='7TYF' size='350' frame='true' side='right' caption='Human AMYR1' scene | + | <StructureSection load='7TYF' size='350' frame='true' side='right' caption='Human AMYR1' scene='10/1038869/Overview-final/1'>Overall structure of the Amylin GPCR bound to Amylin ligand |
This is a default text for your page '''Mathias Vander Eide/Sandbox 1'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | This is a default text for your page '''Mathias Vander Eide/Sandbox 1'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
| Line 17: | Line 17: | ||
== Structural Components == | == Structural Components == | ||
| - | |||
| - | [[Image:7TYF_Overview.png|400 px|right|thumb|Figure 1]] | ||
=== Calcitonin Receptor === | === Calcitonin Receptor === | ||
| - | === RAMP === | + | === RAMP === |
| + | |||
| + | === Peptide === | ||
| + | |||
| + | <scene name='10/1038869/Amylin_peptide-final/1'>Amylin Peptide</scene> | ||
| + | |||
| + | <scene name='10/1038869/Amidated_c-term/1'>Amidated C-Terminus of Amylin Ligand</scene> | ||
| + | |||
| + | <scene name='10/1038869/N-term_disulfide/1'>N-Terminus disulfide bond on Amylin Ligand</scene> | ||
| + | |||
| + | ==== Bypass Motif ==== | ||
| + | |||
| + | The <scene name='10/1038869/Bypass_overview/3'>Bypass Motif</scene> is a series of residues in the midsection of the amylin peptide that are crucial for providing structural specificity for the AMYR. Without RAMP association, the CTR is in a relaxed, fluid state, allowing the binding of calcitonin. When RAMP binds the receptor, it is forced into a new, rigid conformation, which interferes with calcitonin binding. Amylin's bypass motif | ||
| + | |||
| + | |||
| + | <scene name='10/1038869/Bypass_overview/3'>Bypass Motif</scene> | ||
| + | |||
| + | <scene name='10/1038869/Sct_bypass/1'>Calcitonin</scene> | ||
| + | |||
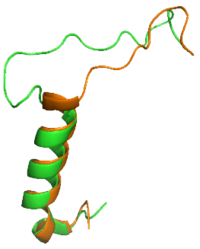
| + | [[Image:SCT_rAmy_Overlay_v2.png|200 px|left|thumb|Overlay of sCT (orange) and rAmy (green)]] | ||
| + | |||
| + | <scene name='10/1038869/Bypass-ecdl_h-bond/4'>Bypass Motif interaction with ECDloop4</scene> | ||
| + | |||
| + | <scene name='10/1038869/Bypass-amyr/3'>Bypass Motif interaction with AMYR</scene> | ||
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
Current revision
Human Amylin Receptor with Associated RAMP1
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
Student Contributors
- Mathias Vander Eide
- Andrew Helmerich
- Ben Whiteside