We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Mathias Vander Eide/Sandbox 1
From Proteopedia
< User:Mathias Vander Eide(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 32: | Line 32: | ||
==== Bypass Motif ==== | ==== Bypass Motif ==== | ||
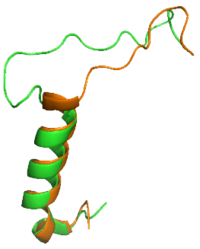
| - | <scene name='10/1038869/Bypass_overview/3'> | + | The <scene name='10/1038869/Bypass_overview/3'>Bypass Motif</scene> is a series of residues in the midsection of the amylin peptide that are crucial for providing structural specificity for the AMYR. Without RAMP association, the CTR is in a relaxed, fluid state, allowing the binding of calcitonin. When RAMP binds the receptor, it is forced into a new, rigid conformation, which interferes with calcitonin binding. Amylin's bypass motif |
| - | <scene name='10/1038869/Sct_bypass/1'> | + | |
| + | <scene name='10/1038869/Bypass_overview/3'>Bypass Motif</scene> | ||
| + | |||
| + | <scene name='10/1038869/Sct_bypass/1'>Calcitonin</scene> | ||
[[Image:SCT_rAmy_Overlay_v2.png|200 px|left|thumb|Overlay of sCT (orange) and rAmy (green)]] | [[Image:SCT_rAmy_Overlay_v2.png|200 px|left|thumb|Overlay of sCT (orange) and rAmy (green)]] | ||
Current revision
Human Amylin Receptor with Associated RAMP1
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
Student Contributors
- Mathias Vander Eide
- Andrew Helmerich
- Ben Whiteside