We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Preston Roa/Sandbox 1
From Proteopedia
< User:Preston Roa(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 31: | Line 31: | ||
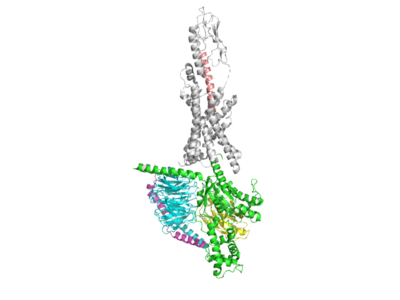
One way in which the Tirzepatide peptide fosters biased signaling is by utilizing particular residues from both GLP-1 and GIP in order to carefully stimulate parts of both pathways. | One way in which the Tirzepatide peptide fosters biased signaling is by utilizing particular residues from both GLP-1 and GIP in order to carefully stimulate parts of both pathways. | ||
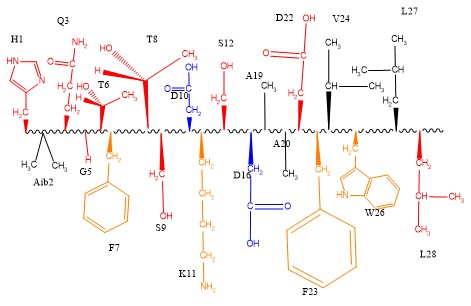
| - | [[Image:Tirzepatide modifications.jpg]] | + | [[Image:Tirzepatide modifications.jpg]]Figure 1. The tirzepatide ligand can be viewed as a combination of both GLP-1 and GIP natural hormones. Shown in red are all the modified residues of tirzepatide. The blue residues are residues that both Tirzepatide and GLP-1 share. |
One way in which the Tirzepatide peptide differs from both GLP-1 and GIP is seen through two mutations at residue sites 2 and 13. These residues have been mutated to mutant AIB residue which is physiologically vital for the performance of the drug as it prevents cleavage from the enzyme DPP-4, which normally cleaves the native GLP-1 peptide <scene name='10/1037513/7rgp-_2aib_mutation/4'>Tirzepatide 2AIB Mutation</scene> <scene name='10/1037513/7rgp_-_13aib_mutation/4'>Tirzepatide 13AIB Mutation</scene>. Tirzepatide binds similarly to the native GLP-1 peptide to its GLP-1R by forming hydrogen bonds with the receptor. Y1 of the Tirzepatide peptide is seen hydrogen bonding with Q234 of the GLP-1R, allowing for the peptide to be anchored to the receptor <scene name='10/1037513/Y1_hbond_w_q234/8'>Tirz Hbond Y1 Q234</scene>. The Tirzepatide drug is furthermore stabilized to the GLP-1R through pi-stacking interactions between Y10 of the drug and Y145 of the receptor <scene name='10/1037513/Pi_stacking_y10_and_y145/5'>Pi Stacking Y10 (Tirzepatide) and Y145 (GLP-1R)</scene>. | One way in which the Tirzepatide peptide differs from both GLP-1 and GIP is seen through two mutations at residue sites 2 and 13. These residues have been mutated to mutant AIB residue which is physiologically vital for the performance of the drug as it prevents cleavage from the enzyme DPP-4, which normally cleaves the native GLP-1 peptide <scene name='10/1037513/7rgp-_2aib_mutation/4'>Tirzepatide 2AIB Mutation</scene> <scene name='10/1037513/7rgp_-_13aib_mutation/4'>Tirzepatide 13AIB Mutation</scene>. Tirzepatide binds similarly to the native GLP-1 peptide to its GLP-1R by forming hydrogen bonds with the receptor. Y1 of the Tirzepatide peptide is seen hydrogen bonding with Q234 of the GLP-1R, allowing for the peptide to be anchored to the receptor <scene name='10/1037513/Y1_hbond_w_q234/8'>Tirz Hbond Y1 Q234</scene>. The Tirzepatide drug is furthermore stabilized to the GLP-1R through pi-stacking interactions between Y10 of the drug and Y145 of the receptor <scene name='10/1037513/Pi_stacking_y10_and_y145/5'>Pi Stacking Y10 (Tirzepatide) and Y145 (GLP-1R)</scene>. | ||
| Line 43: | Line 43: | ||
===Future Directions=== | ===Future Directions=== | ||
| - | The future direction of Tirzapetide and its pharmaceutical uses include further testing of its effects both short term and long term. Current adverse side effects need to be continually monitored, as do all pharmaceuticals. Tirzepatide’s dual action on both GIP and GLP-1 receptors create a greater effect of glucose control and body weight regulation than the body’s natural ligands, however work can continue to be done on a triple-acting agonist. A possible drug technology that is currently in development at Eli Lilly not only effects the GIP and GLP-1 protein receptors, but also glucagon receptors. This promises to be a revolutionary drug for type 2 diabetes and weight loss. | + | The future direction of Tirzapetide and its pharmaceutical uses include further testing of its effects both short term and long term. Current adverse side effects need to be continually monitored, as do all pharmaceuticals. Tirzepatide’s dual action on both GIP and GLP-1 receptors create a greater effect of glucose control and body weight regulation than the body’s natural ligands, however work can continue to be done on a triple-acting agonist. A possible drug technology that is currently in development at Eli Lilly not only effects the GIP and GLP-1 protein receptors, but also glucagon receptors. The drug is known as 'Peptide 20'.This promises to be a revolutionary drug for type 2 diabetes and weight loss. |
| + | |||
| + | [[Image:peptide20.jpg]] | ||
| + | Figure 2. The structure of Peptide 20 will interact with the GLP-1 receptor in many different ways. Shown are several residues and their interactions. Red shows hydrogen bonding, black shows Van der Waals interactions, orange shows pi stacking, and blue shows salt bridges. NOTE: not all residues are shown. | ||
</StructureSection> | </StructureSection> | ||
Current revision
GLP-1
| |||||||||||
References
[1] Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017 Dec 1;127(12):4217-4227. doi: 10.1172/JCI97233. Epub 2017 Dec 1. PMID: 29202475; PMCID: PMC5707151.
[2] Mayendraraj, A., Rosenkilde, M. M., & Gasbjerg, L. S. (2022). GLP-1 and GIP receptor signaling in beta cells - A review of receptor interactions and co-stimulation. Peptides, 151, 170749. https://doi.org/10.1016/j.peptides.2022.170749
[3] Seino, Y., Fukushima, M., & Yabe, D. (2010). GIP and GLP-1, the two incretin hormones: Similarities and differences. Journal of diabetes investigation, 1(1-2), 8–23. https://doi.org/10.1111/j.2040-1124.2010.00022.x
Student Contributors
- Preston Roa
- Jack Guckien
- Sam Reichenbach