User:Letícia Oliveira Rojas Cruz/Sandbox 1
From Proteopedia
< User:Letícia Oliveira Rojas Cruz(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | ==Angiotensin-converting enzyme 2 (ACE2)== | + | ==Angiotensin-converting enzyme 2 (ACE2) - (PDB 1R42)== |
<StructureSection load='6m17' size='425' side='right' caption='PDB 6m17' scene='10/1083732/Ace2_completa/3'> | <StructureSection load='6m17' size='425' side='right' caption='PDB 6m17' scene='10/1083732/Ace2_completa/3'> | ||
| - | <scene name='10/1083732/Ace2_completa/3'>Angiotensin-converting enzyme 2 (ACE2)</scene> is an important protein expressed in several human tissues, such as the heart and kidneys. It plays a key role in the Renin-Angiotensin System - " | + | <scene name='10/1083732/Ace2_completa/3'>Angiotensin-converting enzyme 2 (ACE2)</scene> is an important protein expressed in several human tissues, such as the heart and kidneys. It plays a key role in the Renin-Angiotensin-Aldosterone System - "RAAS" - by catalyzing the conversion of angiotensin II (Ang II), promoting blood pressure regulation. |
| - | Another function of ACE2 is related to SARS-CoV-2 infection, the virus responsible for the COVID-19 pandemic. In this context, ACE2 serves as the entry receptor for the virus. | + | Another function of ACE2 is related to SARS-CoV-2 infection, the virus responsible for the COVID-19 pandemic. In this context, ACE2 serves as the entry receptor for the virus. <ref>PMID:33389262</ref> |
[[Image:Protein ACE2 PDB 1r42.png|225px|right|thumb|Protein ACE2, [[1r42]]]] | [[Image:Protein ACE2 PDB 1r42.png|225px|right|thumb|Protein ACE2, [[1r42]]]] | ||
| Line 20: | Line 20: | ||
== Physiological Function == | == Physiological Function == | ||
| - | <scene name='10/1083732/Ace2_completa/3'>ACE2</scene> is a transmembrane glycoprotein that has an extracellular catalytic domain. It is classified as a hydrolase, more specifically a carboxypeptidase-type peptidase, responsible for the cleavage of peptide bonds at the C-terminal site. | + | <scene name='10/1083732/Ace2_completa/3'>ACE2</scene> is a transmembrane glycoprotein that has an extracellular catalytic domain. It is classified as a hydrolase, more specifically a carboxypeptidase-type peptidase, responsible for the cleavage of peptide bonds at the C-terminal site. <ref>PMID:17897633</ref> |
The ACE2 acts to cleave a single C-terminal residue of some substrates. One of those substrates is Angiotensin II (Ang II), which is degrated into Angiotensin-(1-7); to a lesser extent, Angiotensin I (Ang I) is also a substrate, turning in into Angiotensin-(1-9). | The ACE2 acts to cleave a single C-terminal residue of some substrates. One of those substrates is Angiotensin II (Ang II), which is degrated into Angiotensin-(1-7); to a lesser extent, Angiotensin I (Ang I) is also a substrate, turning in into Angiotensin-(1-9). | ||
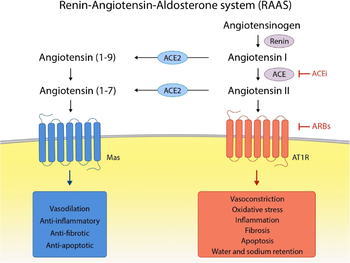
| - | The Renin-Angiotensin System - " | + | The Renin-Angiotensin-Aldosterone System - "RAAS" -, in which ACE2 acts, is an important signaling pathway responsible for vascular homeostasis. |
| - | In general, Ang II, generated by the action of ACE on Ang I, and this one by the action of Renin on Angiotensinogen, acts on AT1 and AT2 receptors. Its main action is vasoconstriction (by AT1 receptor), increasing blood pressure. On the other hand, Angiotensin (1-7), generated from Ang II by ACE2, has the opposite function, leading to a decline in blood pressure through vasodilation and release of Nitric Oxide (NO) through its action on the MAS oncogene receptor, antagonizing the actions of Ang II. | + | In general, Ang II, generated by the action of ACE on Ang I, and this one by the action of Renin on Angiotensinogen, acts on AT1 and AT2 receptors. Its main action is vasoconstriction (by AT1 receptor), increasing blood pressure. On the other hand, Angiotensin (1-7), generated from Ang II by ACE2, has the opposite function, leading to a decline in blood pressure through vasodilation and release of Nitric Oxide (NO) through its action on the MAS oncogene receptor, antagonizing the actions of Ang II. <ref>PMID:19461648</ref> |
| + | |||
| + | [[Image:RAAS.png|350px]] <ref>PMID:33389262</ref> | ||
== Pathological Relevance == | == Pathological Relevance == | ||
The ACE2 protein became better known and widely publicized in 2020 due to its role in the infection by '''SARS-CoV-2''', the virus responsible for the Covid-19 pandemic. | The ACE2 protein became better known and widely publicized in 2020 due to its role in the infection by '''SARS-CoV-2''', the virus responsible for the Covid-19 pandemic. | ||
| - | In this disease, ACE2 acts as a viral receptor, to which the Spike (S) viral protein binds, promoting the entry of the virus into the host cell. The specific region of the S protein responsible for binding to the ACE2 receptor is called '''receptor-binding domain (RBD)''', and is located in the S1 subunit of the Spike protein. | + | In this disease, ACE2 acts as a viral receptor, to which the Spike (S) viral protein binds, promoting the entry of the virus into the host cell. The specific region of the S protein responsible for binding to the ACE2 receptor is called '''receptor-binding domain (RBD)''', and is located in the S1 subunit of the Spike protein. <ref>PMID:32142651</ref> |
| - | == Structure highlights == | + | == Structure highlights<ref>PMID:32132184</ref>== |
=== ACE2-B0AT1 Complex === | === ACE2-B0AT1 Complex === | ||
'''''Association with B0AT1''''' | '''''Association with B0AT1''''' | ||
| Line 51: | Line 53: | ||
=== SARS-CoV-2 Binding === | === SARS-CoV-2 Binding === | ||
'''{{Font color|crimson|ACE2}}''' <scene name='10/1083732/Sars_cov_2/2'>binds</scene> to the '''{{Font color|darkmagenta|Spike (S)}}''' glycoprotein of the SARS-CoV-2 virus, promoting its internalization into the cell. More specifically, binding occurs between the PD subunit of ACE2 and the receptor binding domain (RBD) of the S1 subunit of the S protein. Each PD binds to an RBD by polar bonds, with one ACE2 dimer accommodating two S protein trimers. | '''{{Font color|crimson|ACE2}}''' <scene name='10/1083732/Sars_cov_2/2'>binds</scene> to the '''{{Font color|darkmagenta|Spike (S)}}''' glycoprotein of the SARS-CoV-2 virus, promoting its internalization into the cell. More specifically, binding occurs between the PD subunit of ACE2 and the receptor binding domain (RBD) of the S1 subunit of the S protein. Each PD binds to an RBD by polar bonds, with one ACE2 dimer accommodating two S protein trimers. | ||
| + | </StructureSection> | ||
== References == | == References == | ||
| - | + | <references/> | |
| - | HOFFMANN, M. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, v. 181, n. 2, p. 271–280, mar. 2020. | ||
| - | |||
| - | IWAI, M.; HORIUCHI, M. Devil and angel in the renin–angiotensin system: ACE–angiotensin II–AT1 receptor axis vs. ACE2–angiotensin-(1–7)–Mas receptor axis. Hypertension Research, v. 32, n. 7, p. 533–536, 22 maio 2009. | ||
| - | |||
| - | KEGG ENZYME. angiotensin-converting enzyme 2. Disponível em: <https://www.genome.jp/dbget-bin/www_bget?enzyme+3.4.17.23>. | ||
| - | |||
| - | LAMBERT, D. W.; HOOPER, N. M.; TURNER, A. J. Angiotensin-converting enzyme 2 and new insights into the renin–angiotensin system. Biochemical Pharmacology, v. 75, n. 4, p. 781–786, fev. 2008. | ||
| - | |||
| - | MILLET, J. K.; WHITTAKER, G. R. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology, v. 517, p. 3–8, abr. 2018. | ||
OUDIT, G. Y. et al. Angiotensin-converting enzyme 2—at the heart of the COVID-19 pandemic. Cell, v. 186, n. 5, p. 906–922, mar. 2023. | OUDIT, G. Y. et al. Angiotensin-converting enzyme 2—at the heart of the COVID-19 pandemic. Cell, v. 186, n. 5, p. 906–922, mar. 2023. | ||
| - | |||
| - | YAN, R. et al. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science, v. 367, n. 6485, 4 mar. 2020. | ||
Current revision
Angiotensin-converting enzyme 2 (ACE2) - (PDB 1R42)
| |||||||||||
References
- ↑ Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021 May;40(5):905-919. PMID:33389262 doi:10.1007/s10096-020-04138-6
- ↑ Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 and new insights into the renin-angiotensin system. Biochem Pharmacol. 2008 Feb 15;75(4):781-6. PMID:17897633 doi:10.1016/j.bcp.2007.08.012
- ↑ Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-Mas receptor axis. Hypertens Res. 2009 Jul;32(7):533-6. PMID:19461648 doi:10.1038/hr.2009.74
- ↑ Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021 May;40(5):905-919. PMID:33389262 doi:10.1007/s10096-020-04138-6
- ↑ Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16;181(2):271-280.e8. doi: 10.1016/j.cell.2020.02.052. Epub 2020, Mar 5. PMID:32142651 doi:http://dx.doi.org/10.1016/j.cell.2020.02.052
- ↑ Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020 Mar 4. pii: science.abb2762. doi: 10.1126/science.abb2762. PMID:32132184 doi:http://dx.doi.org/10.1126/science.abb2762
OUDIT, G. Y. et al. Angiotensin-converting enzyme 2—at the heart of the COVID-19 pandemic. Cell, v. 186, n. 5, p. 906–922, mar. 2023.