Sulfite Oxidase
From Proteopedia
(Difference between revisions)
| (24 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
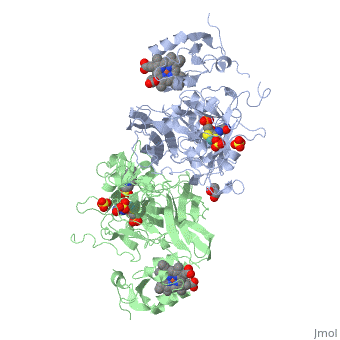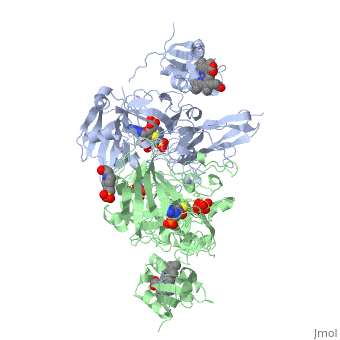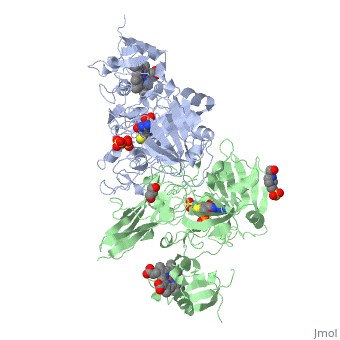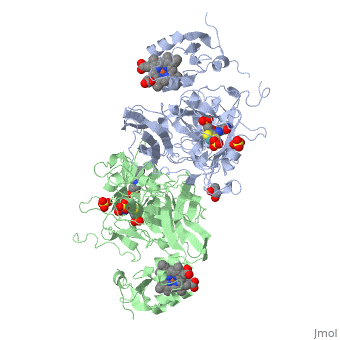
| - | + | <StructureSection load='1sox' size='350' side='right' caption='Chicken sulfite oxidase dimer complex with sulfate, Mo atom (blue-green) protoporphyrin, glycerin, HEPES and inhibitor [[1sox]]'> | |
| - | + | '''Sulfite oxidase''' is a crucial enzyme that is responsible for oxidizing harmful sulfites to sulfates. The enzyme is found in the mitochondria of the cell and mostly can be found in hearts, kidneys and livers. Very little can be seen in the brain, skeletal muscle, blood and spleen. | |
| - | + | ||
| - | + | ||
| - | Sulfite oxidase is a crucial enzyme that is responsible for oxidizing harmful sulfites to sulfates. The enzyme is found in the mitochondria of the cell and mostly can be found in hearts, kidneys and livers. Very little can be seen in the brain, skeletal muscle, blood and spleen. | + | |
The enzyme is a homodimer, a chemical entity consisting of two identical subunits called monomers, which are linked together by intramolecular forces. Sulfite oxidase contains two identical subunits. Each subunit has two domains, iron and molybdenum that are linked together by a loop composed of amino acids. | The enzyme is a homodimer, a chemical entity consisting of two identical subunits called monomers, which are linked together by intramolecular forces. Sulfite oxidase contains two identical subunits. Each subunit has two domains, iron and molybdenum that are linked together by a loop composed of amino acids. | ||
| Line 10: | Line 7: | ||
Point mutations in sulfite oxidase and defects in the molybdenum cofactor synthesis can ultimately lead to sulfite oxidase deficiency. The disorder causes neurological disorders, mental retardation, physical deformities, deterioration of the brain, and ultimately death. | Point mutations in sulfite oxidase and defects in the molybdenum cofactor synthesis can ultimately lead to sulfite oxidase deficiency. The disorder causes neurological disorders, mental retardation, physical deformities, deterioration of the brain, and ultimately death. | ||
| - | |||
==Sulfite Oxidase Electron Transfer== | ==Sulfite Oxidase Electron Transfer== | ||
| - | + | The molybdopertin cofactor is crucial to the enzyme because the oxidation of the sulfite occurs at the molybdenum center; the molybdenum center serves as the active site. When the molybdenum reacts with sulfite, one oxygen atom is transferred to sulfite, which produces sulfate and consequently reduces the molybdenum center by two electrons, thus resulting in Mo(IV). Intramolcular electron transfer from Mo(IV) to Fe(III) results in Fe(II) and Mo(V). One-electron photochemical reduction then moves Fe(II) to the exterior cytochrome resulting in Fe(III). This process occurs twice, and restores the enzyme to its original state. | |
| - | + | ||
| - | The molybdopertin cofactor is crucial to the enzyme because the oxidation of the sulfite occurs at the molybdenum center; the molybdenum center serves as the active site. When the molybdenum reacts with sulfite | + | |
| - | + | ||
| - | + | ||
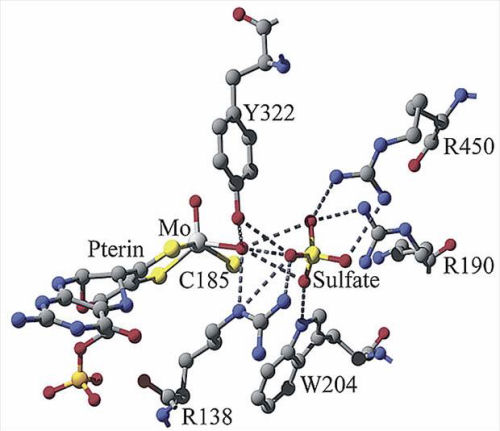
== Tryptophan's Importance in Sulfite Oxidase == | == Tryptophan's Importance in Sulfite Oxidase == | ||
| - | |||
Tryptophan has been shown as a crucial amino acid that can facilitate electron transfer. A paper written by Shih C (Shih C, Museth AK, Abrahamsson M, Blanco-Rodriguez AM, Di Bilio AJ, Sudhamsu J, Crane BR, Ronayne KL, Towrie M, Vlcek A Jr, Richards JH, Winkler JR, Gray HB. Science. 2008. 320, 1760-1762.) suggests that multi-step electron tunneling can enhance charge transfer rates, in which redox active amino acid side chains act as intermediate donors or acceptors. | Tryptophan has been shown as a crucial amino acid that can facilitate electron transfer. A paper written by Shih C (Shih C, Museth AK, Abrahamsson M, Blanco-Rodriguez AM, Di Bilio AJ, Sudhamsu J, Crane BR, Ronayne KL, Towrie M, Vlcek A Jr, Richards JH, Winkler JR, Gray HB. Science. 2008. 320, 1760-1762.) suggests that multi-step electron tunneling can enhance charge transfer rates, in which redox active amino acid side chains act as intermediate donors or acceptors. | ||
| Line 29: | Line 20: | ||
[[Image:Sulfite-oxidase-active-site-500w.jpg]] | [[Image:Sulfite-oxidase-active-site-500w.jpg]] | ||
| + | </StructureSection> | ||
| + | ==3D structures of sulfite oxidase== | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| + | |||
| + | *Sulfite oxidase | ||
| + | |||
| + | **[[1mj4]] – SOX cytochrome b5 domain – human<br /> | ||
| + | **[[1sox]] – cSOX + Mo – chicken<br /> | ||
| + | **[[3hbg]], [[3hc2]], [[3hbq]], [[3r18]], [[3r19]] – cSOX (mutant) + Mo <br /> | ||
| + | **[[2a99]], [[2a9d]] – cSOX catalytic + dimerization domains + Mo<br /> | ||
| + | **[[2a9b]] - cSOX catalytic + dimerization domains (mutant) + Mo<br /> | ||
| + | **[[1ogp]] – SOX + molybdopterin-thio-molybdenum – ''Arabidopsis thaliana''<br /> | ||
| + | **[[1xdq]], [[1xdy]] – SOX residues 45-334 + Mo – ''Escherichia coli'' | ||
| + | |||
| + | *Complex of sulfite oxidase with substrate | ||
| + | **[[3hbp]] - cSOX (mutant) + Mo + sulfite<br /> | ||
| + | **[[2a9a]] - cSOX catalytic + dimerization domains + Mo + sulfate<br /> | ||
| + | **[[2a9c]] - cSOX catalytic + dimerization domains (mutant) + Mo + sulfate<br /> | ||
| + | **[[6y0k]] – SOX + PO4 – ''Thermus thermophilus''<br /> | ||
| + | }} | ||
== References == | == References == | ||
<ref group='xtra'>PMID: 18583608</ref><ref group='xtra'>PMID: 17459792</ref><references group='xtra'/> | <ref group='xtra'>PMID: 18583608</ref><ref group='xtra'>PMID: 17459792</ref><references group='xtra'/> | ||
| - | [[http://www.chem.arizona.edu/enemark/new/ | + | [[http://www.chem.arizona.edu/enemark/new/ Enemark Research Group]] |
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
3D structures of sulfite oxidase
Updated on 07-December-2023
References
- Shih C, Museth AK, Abrahamsson M, Blanco-Rodriguez AM, Di Bilio AJ, Sudhamsu J, Crane BR, Ronayne KL, Towrie M, Vlcek A Jr, Richards JH, Winkler JR, Gray HB. Tryptophan-accelerated electron flow through proteins. Science. 2008 Jun 27;320(5884):1760-2. PMID:18583608 doi:320/5884/1760
- Feng C, Tollin G, Enemark JH. Sulfite oxidizing enzymes. Biochim Biophys Acta. 2007 May;1774(5):527-39. Epub 2007 Mar 20. PMID:17459792 doi:10.1016/j.bbapap.2007.03.006
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Kimberly Meyers, Joel L. Sussman, Jaime Prilusky