User:Tilman Schirmer/Sandbox 125
From Proteopedia
< User:Tilman Schirmer(Difference between revisions)
| Line 7: | Line 7: | ||
<scene name='User:Tilman_Schirmer/Sandbox_125/Hairpin_pre-albumin_fullmodel/1'>Hairpin fullmodel </scene> | <scene name='User:Tilman_Schirmer/Sandbox_125/Hairpin_pre-albumin_fullmodel/1'>Hairpin fullmodel </scene> | ||
| - | <scene name='User:Tilman_Schirmer/Sandbox_125/Hairpin_pre-albumin_fullmodel/2'>Hairpin fullmodel </scene> | + | <scene name='User:Tilman_Schirmer/Sandbox_125/Hairpin_pre-albumin_fullmodel/2'>Hairpin fullmodel </scene> (atom colors) |
| - | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | + | <br><br> |
| + | ''use better example! Note: some side-chains have multiple conformations.''<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
---- | ---- | ||
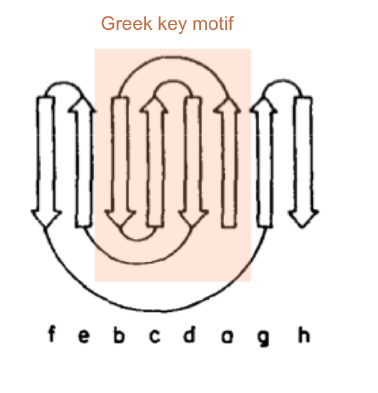
===Greek key topology=== | ===Greek key topology=== | ||
Current revision
β-hairpin
|
Common motif of arrangement of two antiparallel β-strands connected by a regular turn.
(atom colors)
use better example! Note: some side-chains have multiple conformations.
Greek key topology
|
The prealbumin is a β-sandwich composed of an antiparallel and a mixed β-sheet. It forms a with the mixed β- sheets associated to a large transmolecular sheet.
Topology diagram of the monomer:
A consists of 4 antiparallel β-strands in the order bcda, i.e. the the 1st and 4th strand are adjacent and the 2nd and 3rd strand form a β-hairpin.