Mechanosensitive channels: opening and closing
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <applet size='300' frame='true' align='right' caption='Morph of channel opening and closing (see below for details). You may need to scroll up and down to start this animation.' | ||
| - | scene='User:Eric_Martz/Sandbox_0/2oau_2vv5_27-112_morph_pdb_gz/5' /> | ||
==Function== | ==Function== | ||
| + | <StructureSection load='' size='450' side='right' caption='Morph of channel opening and closing (see below for details).' scene='User:Eric_Martz/Sandbox_0/2oau_2vv5_27-112_morph_pdb_gz/5'> | ||
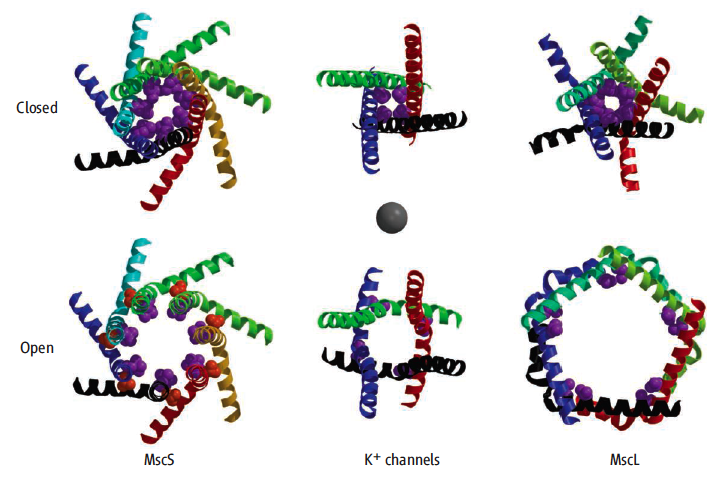
[http://en.wikipedia.org/wiki/Mechanosensitive_ion_channel Mechanosensitive channels] are involved in touch, hearing, and in maintaining osmotic balance. In prokaryotes, they are opened by osmotic stretching of the lipid bilayer in which they are embedded. In eukaroytes, gating involves the cytoskeleton<ref name='vasquez'>PMID:18755978</ref>. Mechanosensitive channels protect bacteria from sudden exposure to fresh water (water with lower [http://en.wikipedia.org/wiki/Osmolarity osmolarity]). Such exposure results in swelling of the bacterial cells due to osmotically-driven water influx. This swelling stretches the lipid bilayers, opening the mechanosensitive channels that are embedded in the inner membrane. The open channels allow rapid release of osmotically active ions or small molecules from the cytoplasm, improving the osmotic balance between the inside and outside of the cells, and preventing rupturing of the cell. | [http://en.wikipedia.org/wiki/Mechanosensitive_ion_channel Mechanosensitive channels] are involved in touch, hearing, and in maintaining osmotic balance. In prokaryotes, they are opened by osmotic stretching of the lipid bilayer in which they are embedded. In eukaroytes, gating involves the cytoskeleton<ref name='vasquez'>PMID:18755978</ref>. Mechanosensitive channels protect bacteria from sudden exposure to fresh water (water with lower [http://en.wikipedia.org/wiki/Osmolarity osmolarity]). Such exposure results in swelling of the bacterial cells due to osmotically-driven water influx. This swelling stretches the lipid bilayers, opening the mechanosensitive channels that are embedded in the inner membrane. The open channels allow rapid release of osmotically active ions or small molecules from the cytoplasm, improving the osmotic balance between the inside and outside of the cells, and preventing rupturing of the cell. | ||
| Line 41: | Line 40: | ||
==Structure of MscS== | ==Structure of MscS== | ||
| + | <!-- | ||
<applet load='2oau' size='400' frame='true' align='right' caption='Closed conformation of E. coli MscS (2oau).' | <applet load='2oau' size='400' frame='true' align='right' caption='Closed conformation of E. coli MscS (2oau).' | ||
| - | scene='User:Eric_Martz/Sandbox_0/2oau/2' /> | + | scene='User:Eric_Martz/Sandbox_0/2oau/2' /> --> |
<!-- | <!-- | ||
--> | --> | ||
| Line 79: | Line 79: | ||
==Opening and Closing== | ==Opening and Closing== | ||
| + | <!-- | ||
<applet size='400' frame='true' align='right' caption='Morph: 2oau to 2vv5.' | <applet size='400' frame='true' align='right' caption='Morph: 2oau to 2vv5.' | ||
| - | scene='User:Eric_Martz/Sandbox_0/2oau_2vv5_morph_cao_pdb_gz/1' /> | + | scene='User:Eric_Martz/Sandbox_0/2oau_2vv5_morph_cao_pdb_gz/1' /> --> |
''You may need to '''<font color='red'>scroll up and down</font>''' to start the animation''<ref>Scrolling to start animations seems to be required in Internet Explorer 7 or Firefox 3 (on Windows XP SP3) and in Safari and Firefox 3 (on OS 10.5). This was observed when Proteopedia was using Jmol applet version 11.6.14.</ref>. | ''You may need to '''<font color='red'>scroll up and down</font>''' to start the animation''<ref>Scrolling to start animations seems to be required in Internet Explorer 7 or Firefox 3 (on Windows XP SP3) and in Safari and Firefox 3 (on OS 10.5). This was observed when Proteopedia was using Jmol applet version 11.6.14.</ref>. | ||
| Line 114: | Line 115: | ||
<!--A conserved pattern of glycine and alanine residues in TM3a of MscS (termed "knobs and holes") is important in gating and also in forming the tightly packed, symmetrical TM3a arrangement seen in the nonconducting form | <!--A conserved pattern of glycine and alanine residues in TM3a of MscS (termed "knobs and holes") is important in gating and also in forming the tightly packed, symmetrical TM3a arrangement seen in the nonconducting form | ||
--> | --> | ||
| + | <!-- | ||
<applet size='400' frame='true' align='right' | <applet size='400' frame='true' align='right' | ||
| - | scene='User:Eric_Martz/Sandbox_0/2oau_aligned_cao_pdb/1' /> | + | scene='User:Eric_Martz/Sandbox_0/2oau_aligned_cao_pdb/1' /> --> |
Although the changes in interchain contacts during opening and closing are of interest, the available models have modest resolution (3.5-3.7 Å) that is insufficient for sidechain positions to be known accurately. In addition, each of the seven chains in [[2oau]] was apparently solved independently: when chains B-G are aligned<ref>Alignments were done with [[DeepView]]'s ''Magic Fit''.</ref>, one at a time, with chain A, the median RMS deviation for alpha carbons is 1.54 Å (range 0.57-1.84). This could lead to variations in interchain contacts that may not be meaningful. The seven chains in 2vv5 were apparently restrained to similar conformations: the median alpha carbon RMS (between chain A and the other six) is only 0.07 Å (range 0.10 - 0.09 Å; sidechain RMS runs about 0.4 Å). | Although the changes in interchain contacts during opening and closing are of interest, the available models have modest resolution (3.5-3.7 Å) that is insufficient for sidechain positions to be known accurately. In addition, each of the seven chains in [[2oau]] was apparently solved independently: when chains B-G are aligned<ref>Alignments were done with [[DeepView]]'s ''Magic Fit''.</ref>, one at a time, with chain A, the median RMS deviation for alpha carbons is 1.54 Å (range 0.57-1.84). This could lead to variations in interchain contacts that may not be meaningful. The seven chains in 2vv5 were apparently restrained to similar conformations: the median alpha carbon RMS (between chain A and the other six) is only 0.07 Å (range 0.10 - 0.09 Å; sidechain RMS runs about 0.4 Å). | ||
| Line 133: | Line 135: | ||
<scene name='User:Eric_Martz/Sandbox_0/2vv5_contacts/1'>Contacts to open conformation chain F channel (27-112) colored by chain</scene> ([[2vv5]]), 30 atoms within 4.0 Å. <scene name='User:Eric_Martz/Sandbox_0/2vv5_contacts/2'>Contacts (in and to chain F) colored by element</scene>. | <scene name='User:Eric_Martz/Sandbox_0/2vv5_contacts/1'>Contacts to open conformation chain F channel (27-112) colored by chain</scene> ([[2vv5]]), 30 atoms within 4.0 Å. <scene name='User:Eric_Martz/Sandbox_0/2vv5_contacts/2'>Contacts (in and to chain F) colored by element</scene>. | ||
--> | --> | ||
| + | |||
| + | </StructureSection> | ||
==3D structures of mechanosensitive channel== | ==3D structures of mechanosensitive channel== | ||
Revision as of 14:23, 4 March 2013
Contents |
Function
| |||||||||||
3D structures of mechanosensitive channel
See Also
- Mechanosensitive Channels, the November 2008 article in the Molecule of the Month series.
- Theoretical models of closed, intermediate, and open forms of the MscL channel at 2oar.
- For additional information, see: Membrane Channels & Pumps
Notes and References
- ↑ 1.0 1.1 1.2 Vasquez V, Sotomayor M, Cordero-Morales J, Schulten K, Perozo E. A structural mechanism for MscS gating in lipid bilayers. Science. 2008 Aug 29;321(5893):1210-4. PMID:18755978 doi:http://dx.doi.org/321/5893/1210
- ↑ Gandhi CS, Rees DC. Biochemistry. Opening the molecular floodgates. Science. 2008 Aug 29;321(5893):1166-7. PMID:18755963 doi:http://dx.doi.org/321/5893/1166
- ↑ 3.0 3.1 3.2 3.3 MscS: MechanoSensitive Channel of Small conductance.
- ↑ MscL: MechanoSensitive Channel of Large conductance.
- ↑ Anishkin A, Sukharev S. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys J. 2004 May;86(5):2883-95. PMID:15111405 doi:http://dx.doi.org/10.1016/S0006-3495(04)74340-4
- ↑ 6.0 6.1 The model with the lipid bilayer surfaces represented by pseudoatoms was generated by the server for Orientations of Proteins in Membranes, specifically OPM for 2oau. The resulting file uploaded to Proteopedia is Image:2oau opm.pdb.gz.
- ↑ Residues in the crystallized protein that lack coordinates are indicated by "---" in the SEQRES to coordinates alignment.
- ↑ Evolutionary conservation was calculated by ConSurfDB. See Conservation, Evolutionary.
- ↑ Scrolling to start animations seems to be required in Internet Explorer 7 or Firefox 3 (on Windows XP SP3) and in Safari and Firefox 3 (on OS 10.5). This was observed when Proteopedia was using Jmol applet version 11.6.14.
- ↑ 10.0 10.1 10.2 Wang W, Black SS, Edwards MD, Miller S, Morrison EL, Bartlett W, Dong C, Naismith JH, Booth IR. The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science. 2008 Aug 29;321(5893):1179-83. PMID:18755969 doi:http://dx.doi.org/321/5893/1179
- ↑ 11.0 11.1 The morph of E. coli MscS was generated by the Yale Morph Server (beta.cgi) using 2oau and 2vv5. For animation in Jmol, all atoms except the alpha carbons were deleted to reduce the file size, leaving Image:2oau 2vv5 morph cao.pdb.gz. For the morph of the isolated channel, residues 113-281 were deleted from each of the seven chains, in the closed (2oau) and open (2vv5) models, before submitting them to Yale. The resulting file is Image:2oau 2vv5 27-112 morph.pdb.gz.
- ↑ Beckstein O, Sansom MS. The influence of geometry, surface character, and flexibility on the permeation of ions and water through biological pores. Phys Biol. 2004 Jun;1(1-2):42-52. PMID:16204821 doi:http://dx.doi.org/10.1088/1478-3967/1/1/005
- ↑ Alignments were done with DeepView's Magic Fit.
Proteopedia Page Contributors and Editors (what is this?)
Eric Martz, Jaime Prilusky, Michal Harel, Alexander Berchansky, David Canner