Morphs
From Proteopedia
| |||||||
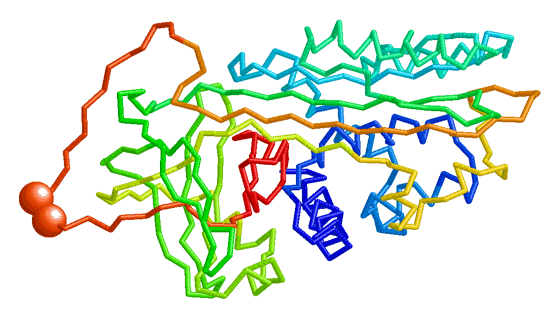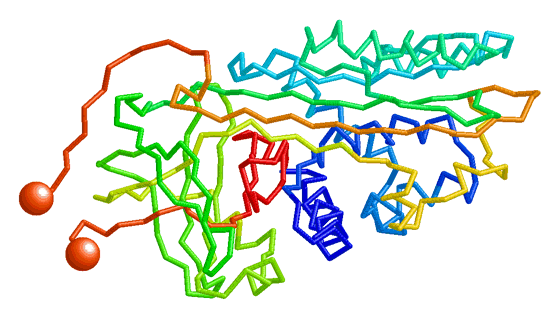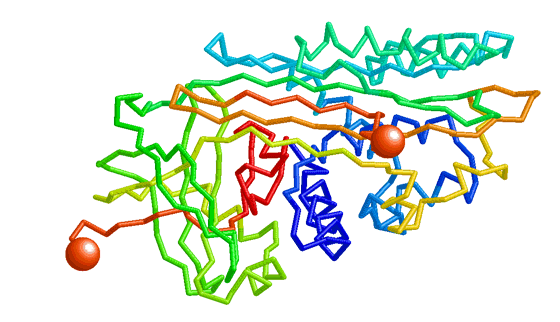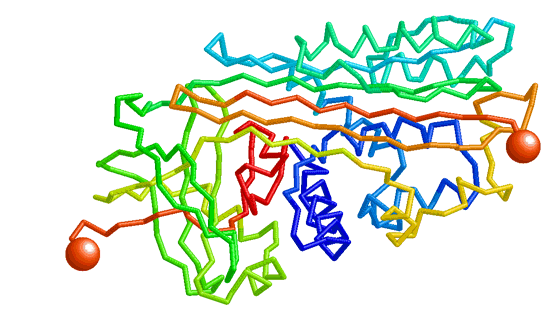
| Morph of the lac repressor complexed with DNA showing the differences between non-specific binding (straight DNA) vs. specific recognition of the operator sequence (kinked DNA). Whether the binding kinks the DNA, or simply stabilizes a pre-existing kink, is unknown. Details. |
A morph is an animation showing a transition between two molecular conformations.
Contents
|
Examples of Morphs
Morphs in Proteopedia
- Coronavirus: SARS-CoV-2 spike protein fusion transformation, a morph animation.
- Coronavirus: SARS-CoV-2 protein S priming by furin, a morph animation.
- Lac repressor morphs the bending of the DNA operator as the repressor protein goes from non-specific to sequence-specific binding. This is the example shown above here.
- Mechanosensitive channels: opening and closing
- Flaps Morph for HIV Protease

- Avian Influenza Neuraminidase, Tamiflu and Relenza shows a morph of the induced fit of N1 to Tamiflu.
- Ribosomal A Site Binding Paromomycin: A Morph
- Citrate synthase: open to closed form
- Pyruvate phosphate dikinase with a true-movie morph of the catalytic reaction and conformational changes.
- Enzyme I of the Phosphoenolpyruvate:Sugar Phosphotransferase System with a true-movie morph of the catalytic reaction and conformational changes.
- Proton Channels includes a morph of a transmembrane channel opening and closing.
- Recoverin, a calcium-activated myristoyl switch includes a morph of the calcium-activated conformational change that expels the myristate from within a protein domain, converting the protein from soluble to membrane anchored with consequent redistribution of its activity.
- Human DEAD-box RNA-helicase DDX19 in the prehydrolysis state with mRNA and ATP bound transitioning to the structure in the post-hydrolysis state with ADP bound reveals an N-terminal extension inserts between the conserved helicase domains to negatively regulate ATPase activity.
- Human alpha-galactosidase bound and unbound form.
- Homing endonuclease I-Ppo binding and cleaving DNA.
- A-DNA to B-DNA Morph
- R vs. L forms of the flagellar filament of bacteria.
- T7 RNA Polymerase
- Lipase lid morph
- Hexokinase#Conformational_change_associated_with_substrate_binding
- Calmodulin#Calmodulin_in_Motion
- Human lactoferrin
Morphs Elsewhere
- Database of Macromolecular Movements with Associated Tools for Flexibility and Geometric Analysis, molmovdb.org, developed by Mark Gerstein and coworkers at Yale University, USA.[1][2]
- Protein Explorer developed by Eric Martz at the University of Massachusetts, Amherst, USA.[3] Note that Protein Explorer went out of service in the early 2000's because it was built around Chime. Chime was not open source, and its owners ceased to maintain it.
- Database of the Morphit Pro server [1]. and associated DynDom software [2] by Steven Hayward and coworkers at the University of East Anglia, UK.
Non-Morph Animations
Why Morph?
The purpose of molecular morphing is to smooth the visual transition between two molecular conformations, making it easier to see and understand the structural differences between them. In contrast, predicting the actual trajectory through which a conformational change occurs is rarely, if ever, the goal of a morph.
Some proteins perform their functions without major conformational changes. On the other hand, some proteins must undergo major changes in secondary, tertiary, or quaternary structure in order to perform their functions. In quite a few cases, investigators have succeeded in obtaining empirically determined structures for a protein in two or more conformations. The challenge for visualization is then to be able to follow the changes in each region between the two conformations.
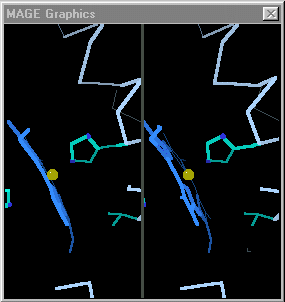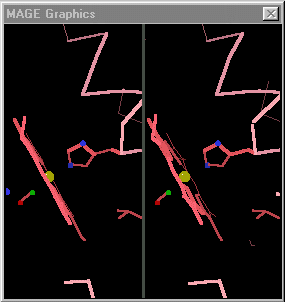
When the differences are small, simply toggling an image between the two states is adequate. David Richardson's MAGE[4], first available in 1992[5] supports visual toggling between macromolecular conformations. Hundreds of interactive molecular structure tutorials called kinemages take advantage of this capability. At the right are snapshots of hemoglobin toggled in MAGE. (MAGE is available in java applet form, and is an option for molecular displays in Proteopedia[6].)
On the other hand, when the conformational changes are large, toggling between two very different conformations leaves the eye unable to grasp what has happened. This is when morphing is useful for seeing and understanding the differences between conformations. A dramatic example is Recoverin.
History of Macromolecular Morphing
Perhaps the first macromolecular morph was in 1995 by Vonrhein, Schlauderer & Schultz[7] who animated substrate binding to nucleoside monophosphate kinases using linear interpolation.
About 1997, Mark Gerstein, Werner Krebs and their team at Yale University released a Morph Server that made it easy to obtain chemically possible morphs. They and others have subsequently used the results of this server to classify molecular movements, and to develop an impressive Database of Macromolecular Movements, molmovdb.org, with research-quality analysis tools and multiple visualization options.[1]
In 1998, encouraged by Joel Sussman, Eric Martz released a Protein Morpher website[8] which displayed morphs of several molecules using the MDL Chime browser plugin[5]. A few years later, he incorporated morph animation capability into Protein Explorer[3], where he implemented a number of additional morphs.
About 2004, Karsten Theis, Craig Martin and coworkers released several morphs that were unusual because they involved hand-modeled intermediate key frames, and in one case[9], proposed the actual trajectory of the conformational change.
Visualizing Morphs
There are two main ways to show macromolecular morphs: true movies, and animations in molecular visualization software[10], such as Jmol, PyMol, etc. Since Proteopedia uses Jmol, we'll mention Jmol in the discussion below.
True Movies
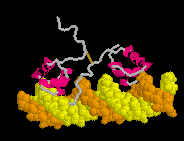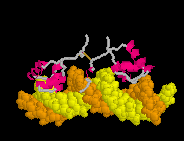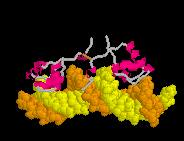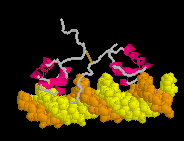 | |
| True movie of the morph shown in Jmol elsewhere near the top of this page. (Stops after 100 cycles; Shift-Reload this page to restart this movie.) |
A true movie is a series of static snaphots displayed sequentially in rapid succession. An example of a true movie of a morph is shown at right. Note that you cannot rotate the molecule with the mouse, so you can view the morph from only the single perspective chosen by the author of the movie. Also, a separate movie must be provided for each change in rendering or coloring, while the rendering and coloring in Jmol can be easily be changed with script commands while displaying the same morph PDB file.
When are true movies advantageous over animations in Jmol? First, if a movie is already available showing the desired morph, using it will save you the time of creating your own morph. This is especially true when generating the atomic coordinates to show the conformational change would be unusually challenging, such as in these true movies of recombined DNA moving through Holliday junctions. Second, if you wish to show the morph in a Powerpoint® slide, a true movie can be pasted right into the slide, while an animation in Jmol is technically quite fussy to install in Powerpoint, and can be done only in Windows, not in Mac OS X.
Methods for making true movies from multiple-model PDB files, e.g. morphs, include:
- Polyview-3D, a free server that works from menus, checkboxes and forms, generating your true movie in publication quality with PyMOL. You don't have to license or install PyMOL or learn how to use it. This is the easiest way. The resulting true movie can be dropped into a Powerpoint® slide.
- eMovie, a storyboard-based tool for making molecular movies. eMovie is a plugin for PyMOL, and thus is best suited to people who are familiar with PyMOL, or wish to become familiar with it. eMovie provides much more powerful moviemaking features than does Polyview-3D, and should be relatively simple to use for any PyMOL-user.
Animations in Jmol
| |||||||
| Morph in Jmol of the lac repressor complexed with DNA. |
There are several reasons why animations in Jmol (such as the one at the top of this page) are advantageous, compared to true movies. First, the morph in Jmol can be viewed from any perspective, while animating, by rotating it with the mouse. It can also be zoomed to examine details. Second, the display and color scheme can be changed easily by sending scripts to Jmol. Also, portions of the molecule can be hidden. In Proteopedia, these latter changes can be done with green links or buttons, as shown:
-OR-
Morphing Methods
All morphs begin with at least two, usually empirical, atomic coordinate files that represent different conformations of the same macromolecule. In some cases, more than two are available[7]. Simple morphs then involve calculating a series of interpolated intermediate conformations using one of the methods below.
Before making a new morph from published PDB files, search the Gallery of Morphs where you will find thousands of morphs from jobs previously submitted to the Yale Morph Server (see below). It is quite possible that your morph has already been made, and is already available there!
Modeling Intermediate Key Frames
In some cases where very large domain movements occur, or where the authors are proposing the trajectory that actually occurs, it is necessary to model intermediates in which portions of the molecule are positioned by hand. Interpolations are then typically calculated between the initial empirical conformation and the first key frame, between intermediate key frames, and finally between the last key frame and the final empirical conformation. Examples of such morphs are those by Karsten Theis, Craig Martin, and Peng Gong concerning T7 RNA polymerase, and inhibition of trypsin by serpin[11].
Chemically Possible Intermediates
Chemically possible (also sometimes called chemically plausible, semi-plausible, or chemically reasonable) means that, at the least, correct covalent bond lengths and angles are maintained, and that atoms are constrained not to pass through each other. This type of morph requires sophisticated software, but luckily such software is freely available and easy to use.
A minor disadvantage of energy-minimized morphs is that the movement in the animation may be rather jerky. In some cases the molecule resists the transition, so moves slowly at first, then rapidly and "uncomfortably" through the mid-point arriving quickly near the final configuration. In contrast, linear interpolation alone (see next section) produces a smooth animation which some people prefer.
Proteopedia PyMOL Morpher
Recommended. Proteopedia provides a page http://proteopedia.org/cgi-bin/morph that sends a morph request to the PyMOL program and automatically uploads the resulting multi-model file to proteopedia. An example of a morph made by the server is here, and it is shown when you click the green links on this page.
Advantages:
- Accepts pre-aligned models so you can control the structural alignment. Or the server will align the models for you.
- The morph can include multiple protein chains, provided that matching chains have the same chain ID names.
- The protein sequences do not need to be identical.
- Some differences in chain length are tolerated.
FATCAT, Godzik Lab
The FATCAT Structural Alignment Server also produces "chemically possible" morphs. After an optionally flexible alignment (permitting twists at hinge points determined by FATCAT), a linear interpolation is done between the aligned models. Then "the intermediate structures are optimized by energy gradient minimization employing a reduced representation force field."
Limitations: FATCAT does only one chain from each model. Chains are re-numbered so it is time consuming to relate the new numbers to the original sequence numbers. In the resulting morph, it appears that some changes may be made to at least one model even when a "rigid" alignment is specified.
Biomolecular Morphing by Kleywegt at Uppsala
Another way to make "chemically reasonable" morphs is available from the Biomolecular Morphing site by Gerard J. Kleywegt at Uppsala University, Sweden. Unlike the Yale server, this is not a server where you can submit a job and get back finished results automatically. Kleywegt's LSQMAN software is downloadable for use on linux, and is free for academic and non-profit use.
Yale Morph Server
NOT WORKING AGAIN in January 2023. STILL NOT WORKING November 2024.
WORKING AGAIN in February, 2021. Their animation player doesn't work because it still requires Java, but you can download the morph PDB file and animate it in Proteopedia or Jmol. See instructions below.
The Yale Morph Server from Mark Gerstein's group. In addition to the chemically possible nature of the results, this server is very attractive because it totally automates both the creation of the multiple-model morph PDB data file, and also its visualization. The server does the structural alignment of the starting PDB files, and can handle modest differences in sequence between the initial and final PDB files. It then does a linear interpolation followed by some energy minimization to render each frame chemically possible.
Morph2 (version 2) of the Yale Morph Server allows you to pre-align (or have the server align) your structures, and gives you the option of minimizing, or not.
Linear Interpolation
In this method, a series of intermediate models is created in which each atom moves in a straight line from is initial position to its final position. Linear interpolation by itself (without any subsequent energy minimization) is relatively easy to calculate, and often suffices, but also has a number of limitations. When animated, the interpolated intermediate conformations often greatly help in visualizing the differences between the initial and final empirical models. However, bond lengths and angles become unrealistic, domains may artifactually shrink, expand, or distort, and chains may even pass through each other during the interpolated movements. When there are substantial changes in secondary structure or large movements of domains, these artifacts make the eye unable to follow the details of the conformational change, and linear interpolation becomes unsatisfactory.
The presence of obvious artifacts of linear interpolation, such as objects passing through each other, can be useful because it reminds viewers that the morph's value is only to help visualize the details of the conformational change -- and is not intended to suggest the actual trajectory of change. An example is the morph of Recoverin, in which the N-linked myristoyl group passes through a protein chain.
Linear Morph Server
User:Karsten Theis has provided a server for linear interpolation between two PDB files: Linear morph between two PDB files. Check the results as it has not yet been completely tested with all situations.
MS-DOS morph2.exe
A very simple program, named morph2.exe, performs linear interpolation and has been freely available since the late 1990's in the PDBTools package by Eric Martz.[12]. This program, which was used for most of the morphs made by Martz (including the Lac repressor morph near the top of this page), requires that the two starting PDB files contain exactly the same atoms in exactly the same order. (Achieving this usually requires some hand editing of the PDB files.) Its advantages include that it is straightforward to include ligand in the morph, or at the end of the morph, and to include multiple protein and nucleic acid chains. Step by step instructions are available within Protein Explorer. While these instructions were written for Chime, rather than Jmol, everything there applies equally to Jmol except the last section on Playback Scripts. Caution: check the results -- morph2.exe appears to fail with very large PDB files but its limits have not been defined.
Combination of rigid body movement and linear interpolation
To explore conformational changes on the fly within Jmol, the storymorph suite defines several Jmol functions to superimpose and morph related structures. The method is described in detail at Jmol/Storymorph, together with some demonstrations and interactive examples.
Animating Morph PDB Files
If you upload your multiple-model morph PDB file to Proteopedia, animating it is as simple as checking that option in Proteopedia's Scene authoring tools.
For those who wish to make their own animation scripts in Jmol outside of Proteopedia (e.g. in the Jmol Tutorial-Authoring Template), the script required to animate a multiple-model PDB file in Jmol is much simpler than what was needed in Chime. Here are the commands needed in Jmol:anim mode palindrome; anim on; anim fps 8; # frames per secondNote that these commands are NOT needed in Proteopedia, and that even if applied, the animation will not be maintained when Proteopedia saves Jmol's state script. Rather, for Proteopedia, use the animation checkbox in the Scene Authoring Tool.
Prior to saving the animation in Proteopedia, or to animating with the above three commands, the models should be displayed and colored as desired. See also the Jmol command script reference manual, available from jmol.org or, in 2008, at this direct link to the Jmol Scripting Documentation.
References, Links, and Notes
- ↑ 1.0 1.1 The Database of Macromolecular Motions: new features added at the decade mark. Flores, S. et al., Nucleic Acids Res., .34 (Database issue) D1–D6. 2006. PubMed 16381870, PDF.
- ↑ Database of Macromolecular Movements with Associated Tools for Flexibility and Geometric Analysis, molmovdb.org, developed by Mark Gerstein and coworkers at Yale University, USA.
- ↑ 3.0 3.1 Protein Explorer: easy yet powerful macromolecular visualization. Martz, E., Trends Biochem Sci. 27:107-9. 2002. PubMed 11852249 developed by Eric Martz at the University of Massachusetts, Amherst, USA.
- ↑ Kinemages by David and Jane Richardson, Duke University, USA.
- ↑ 5.0 5.1 History of Macromolecular Visualization
- ↑ For sample pages that use the MAGE applet in Proteopedia, see Hemoglobin and Ribulose-1,5-bisphosphate carboxylase/oxygenase
- ↑ 7.0 7.1 Movie of the structural changes during a catalytic cycle of nucleoside monophosphate kinases. Vonrhein, Schlauderer & Schultz, Structure 3:483-90, 1995. PubMed 7663945
- ↑ The Protein Morpher website is difficult to view today because it requires obsolete software, and has not been maintained. It was later superceded by the ability of Protein Explorer to display morphs (morphs.proteinexplorer.org).
- ↑ Vonrhein C, Schlauderer GJ, Schulz GE. Movie of the structural changes during a catalytic cycle of nucleoside monophosphate kinases. Structure. 1995 May 15;3(5):483-90. PMID:7663945
- ↑ Molecular visualization software is listed in two categories, free and commercial, in the World Index of Molecular Visualization Resources, MolVisIndex.Org.
- ↑ Theis K, Gong P, Martin CT. Topological and conformational analysis of the initiation and elongation complex of t7 RNA polymerase suggests a new twist. Biochemistry. 2004 Oct 12;43(40):12709-15. PMID:15461442 doi:http://dx.doi.org/10.1021/bi0486987
- ↑ PDBTools by Eric Martz include a simple linear interpolation morphing program. This program operates only in MS-DOS (under MS Windows), but the C source code is included. Available within Protein Explorer are step by step instructions for making linear interpolation morphs.
Proteopedia Page Contributors and Editors (what is this?)
Eric Martz, Wayne Decatur, Karsten Theis, Joel L. Sussman, Angel Herraez, David Canner, Eran Hodis
DOI: https://dx.doi.org/10.14576/100862.1393681 (?)Citation: Martz E, Hodis E, Decatur W, Canner D, 2012, "Morphs",