Interferon
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
[[Image:InterferonSignalingPathway.png|525px|right|thumb|Interferon Pathway<ref>[http://www.jbc.org/content/282/28/20045.full?sid=cbf08059-44d4-4957-8ea7-0351cab9c2ac] Samuel, C.E. "Interferons, Interferon Receptors, Signal Transducer and Transcriptional Activators, and Inteferon Regulatory Factors." ''J Biol Chem'' 2007 282: 20045-20046. First Published on May 14, 2007, doi:10.1074/jbc.R700025200</ref>]] | [[Image:InterferonSignalingPathway.png|525px|right|thumb|Interferon Pathway<ref>[http://www.jbc.org/content/282/28/20045.full?sid=cbf08059-44d4-4957-8ea7-0351cab9c2ac] Samuel, C.E. "Interferons, Interferon Receptors, Signal Transducer and Transcriptional Activators, and Inteferon Regulatory Factors." ''J Biol Chem'' 2007 282: 20045-20046. First Published on May 14, 2007, doi:10.1074/jbc.R700025200</ref>]] | ||
| - | + | ||
| - | + | ||
| Line 8: | Line 7: | ||
<StructureSection load='2hym' size='500' side='right' caption='Click on the green links to the left to view the structural aspects of interferons. PDB ID: [[2hym]])' scene='Interferons/Interferonaandreceptor/2'> | <StructureSection load='2hym' size='500' side='right' caption='Click on the green links to the left to view the structural aspects of interferons. PDB ID: [[2hym]])' scene='Interferons/Interferonaandreceptor/2'> | ||
| - | == | + | ==Type I== |
| + | Type I interferons are homologous helical cytokines that effect a wide variety of cells pleiotropically. These effects range from antiviral activity to antibacterial, antiprozoal, immunodulatory, and cell growth regulatory functions. Without Type I interferons, the survival of the higher vertebrates would be impossible. Because of their strong antiviral and antiproliferative effects, these interferons are used in the treatment of numerous cancers, hepatitis C, and multiple sclerosis. | ||
| - | ==Interferon-β== | + | All type I interferons bind to a cell surface receptor consisting of two subunits: IFNAR1 and IFNAR2. These receptors belong to a class II helical cytokine receptor family (HCRII). Other members of this family include the interferon-γ receptor (IFNGR), tissue factor (TF), the interleukin 10 receptor (IL20R1 and IL20R2), IL-28BP, IFNLR, and IL28Rα.<ref>PMID:17001036</ref> |
| + | |||
| + | ===Interferon-α=== | ||
| + | |||
| + | ===Interferon-β=== | ||
<scene name='Multiple_sclerosis/Interferon_beta/9'>Interferon-β</scene> is a protein growth factor that stimulates an antiviral defense. Its encoding gene is one of only two known vertebrate structural genes that lacks introns.<ref name="Biochem Text">Voet, D., Voet, J.G., and C. Pratt. ''Fundamentals of Biochemistry'' 3rd Edition. Hoboken, NJ: John Wiley and Sons, 2008. Print.</ref> | <scene name='Multiple_sclerosis/Interferon_beta/9'>Interferon-β</scene> is a protein growth factor that stimulates an antiviral defense. Its encoding gene is one of only two known vertebrate structural genes that lacks introns.<ref name="Biochem Text">Voet, D., Voet, J.G., and C. Pratt. ''Fundamentals of Biochemistry'' 3rd Edition. Hoboken, NJ: John Wiley and Sons, 2008. Print.</ref> | ||
Interferon-β is a relatively simple biological response modifier, with several <scene name='Multiple_sclerosis/Interferon_beta_labeled/1'>identifiable regions</scene>. It consists of five <scene name='Multiple_sclerosis/Ifnb_helices_in_color/1'>alpha helices</scene>, as well as multiple interconnecting <scene name='Multiple_sclerosis/Interferon_beta_loops/2'>loop regions</scene>. Helices A, B and D run <scene name='Multiple_sclerosis/Ifnb_parallel_abd/3'>parallel to one another</scene>, and helices C and E run <scene name='Multiple_sclerosis/Ifnb_antiparallel/1'>anti-parallel</scene> to the other three helices, but <scene name='Multiple_sclerosis/Ifnb_antiparallel_ce/3'>parallel</scene> to one another. Helix A consists of residues 6-23; Helix B consists of residues 49-65; Helix C consists of residues 77-91; Helix D consists of residues 112-131; and Helix E consists of residues 135-155.<ref name="Structure Ifn B">PMID:20616576</ref><ref name="UniProt">http://www.uniprot.org/uniprot/P00784</ref> | Interferon-β is a relatively simple biological response modifier, with several <scene name='Multiple_sclerosis/Interferon_beta_labeled/1'>identifiable regions</scene>. It consists of five <scene name='Multiple_sclerosis/Ifnb_helices_in_color/1'>alpha helices</scene>, as well as multiple interconnecting <scene name='Multiple_sclerosis/Interferon_beta_loops/2'>loop regions</scene>. Helices A, B and D run <scene name='Multiple_sclerosis/Ifnb_parallel_abd/3'>parallel to one another</scene>, and helices C and E run <scene name='Multiple_sclerosis/Ifnb_antiparallel/1'>anti-parallel</scene> to the other three helices, but <scene name='Multiple_sclerosis/Ifnb_antiparallel_ce/3'>parallel</scene> to one another. Helix A consists of residues 6-23; Helix B consists of residues 49-65; Helix C consists of residues 77-91; Helix D consists of residues 112-131; and Helix E consists of residues 135-155.<ref name="Structure Ifn B">PMID:20616576</ref><ref name="UniProt">http://www.uniprot.org/uniprot/P00784</ref> | ||
| + | |||
| + | ===Interferon-ε=== | ||
| + | ===Interferon-κ=== | ||
| + | ===Interferon-ω=== | ||
| + | |||
| + | ==Type II== | ||
==Interferon-γ== | ==Interferon-γ== | ||
| + | |||
</StructureSection> | </StructureSection> | ||
Revision as of 15:16, 22 April 2012
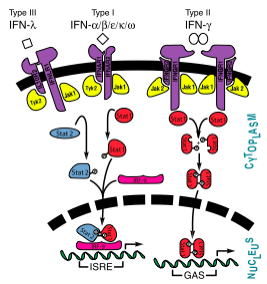
Interferon Pathway[1]
| |||||||||||
A comparative representation of three interferons
|
|
|
Synchronize the three applets showing interferons alpha, beta, and gamma by clicking the checkbox
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Kirsten Eldredge, Alexander Berchansky, Joel L. Sussman, Karl Oberholser, Jaime Prilusky
