This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 475
From Proteopedia
(→'''Structure''') |
|||
| Line 5: | Line 5: | ||
== <span style="font-size:120%">ACETYLCHOLINE RECEPTOR</span> == | == <span style="font-size:120%">ACETYLCHOLINE RECEPTOR</span> == | ||
<Structure load='2bg9' size='350' frame='true' align='right' | <Structure load='2bg9' size='350' frame='true' align='right' | ||
| - | caption='Refined nicotinic receptor structure at 4.0 Å resolution ([[2BG9]]). 5 chain structure of | + | caption='Refined nicotinic receptor structure at 4.0 Å resolution ([[2BG9]]). 5 chain structure of ion channels with sequence from [http://en.wikipedia.org/wiki/Marbled_electric_ray Torpedo marmorata].' scene='' /> |
<span style="font-size:110%"> | <span style="font-size:110%"> | ||
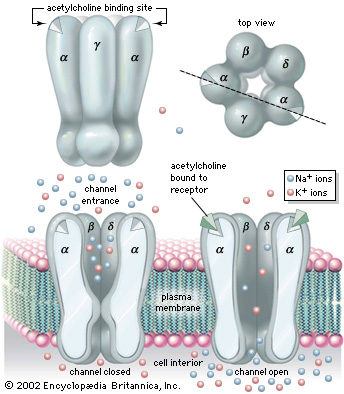
'''Nicotinic Acetylcholine Receptor''' (nAChRs) is a neurotransmitter receptor also known as a specialized integral membrane protein. Integral membrane proteins are permanently bound to the phospholipid bilayer. They provide a communication pathway between the internal and external environments of the cell. Acetylcholine receptor proteins reside as ligand-gated ion channels on the motor end plate of the postsynaptic cleft in the neuromuscular junction. | '''Nicotinic Acetylcholine Receptor''' (nAChRs) is a neurotransmitter receptor also known as a specialized integral membrane protein. Integral membrane proteins are permanently bound to the phospholipid bilayer. They provide a communication pathway between the internal and external environments of the cell. Acetylcholine receptor proteins reside as ligand-gated ion channels on the motor end plate of the postsynaptic cleft in the neuromuscular junction. | ||
</span> | </span> | ||
| + | |||
<span style="font-size:110%"> | <span style="font-size:110%"> | ||
'''nAChRs''' as an ionotropic receptor, which are in direct facilitation of ion movement. When a ligand binds to the active site of the receptor, the channel responds by opening. Likewise, when the ligand is removed the channel closes. They are activated by the binding of the acetylcholine neurotransmitter. Once the receptor is activated the ion channel opens allowing the exchange of salt ions. Nicotinic acetylcholine receptors are responsible for the regulation of sodium (Na+) ion concentration entering and potassium (K+) ion concentration leaving the muscular cytosol, ultimately resulting in an action potential from the differences in electrochemical gradients. | '''nAChRs''' as an ionotropic receptor, which are in direct facilitation of ion movement. When a ligand binds to the active site of the receptor, the channel responds by opening. Likewise, when the ligand is removed the channel closes. They are activated by the binding of the acetylcholine neurotransmitter. Once the receptor is activated the ion channel opens allowing the exchange of salt ions. Nicotinic acetylcholine receptors are responsible for the regulation of sodium (Na+) ion concentration entering and potassium (K+) ion concentration leaving the muscular cytosol, ultimately resulting in an action potential from the differences in electrochemical gradients. | ||
</span> | </span> | ||
| + | |||
<span style="font-size:110%"> | <span style="font-size:110%"> | ||
| Line 25: | Line 27: | ||
== '''Structure''' == | == '''Structure''' == | ||
| - | [[Image:Nicotinic Acetylcholine Receptor.jpg |left|alt=text|Caption]] | + | [[Image:Nicotinic Acetylcholine Receptor.jpg |left|375px|alt=text|Caption]] |
<span style="font-size:110%"> | <span style="font-size:110%"> | ||
| - | Nicotinic Acetylcholine Receptor is composed up five distinct subunits (shown in figure to left): α1, α2, β1, δ, and ε at approximately a molecular mass of 290 kDa. The subunits are symmetrically arranged around a central pore. Each subunit is a transmembrane domain with the N-terminus and C-terminus located on the extracellular side of the plasma membrane. Each subunit are composed of long protein chains that extend across the cell membrane. The subunits have a composition of around ~20% beta sheets and ~40% alpha helices from about ~370 residues in total. | + | Nicotinic Acetylcholine Receptor is composed up '''five distinct subunits''' (shown in figure to left): '''α1''', '''α2''', '''β1''', '''δ''', and '''ε''' at approximately a molecular mass of 290 kDa. The subunits are symmetrically arranged around a central pore. Each subunit is a transmembrane domain with the N-terminus and C-terminus located on the extracellular side of the plasma membrane. Each subunit are composed of long protein chains that extend across the cell membrane. The subunits have a composition of around '''~20%''' beta sheets and '''~40%''' alpha helices from about ~370 residues in total. |
</span> | </span> | ||
| + | |||
<span style="font-size:110%">[[Image:2bg9 composite.gif |right|130px|alt=text|Caption]] | <span style="font-size:110%">[[Image:2bg9 composite.gif |right|130px|alt=text|Caption]] | ||
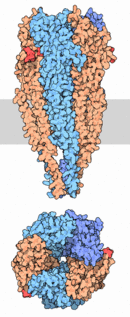
| - | In vertebrates, nicotinic receptors are classified by their main sites of expression into two subtypes: muscle-type nicotinic receptors and neuronal-type nicotinic receptors. In the muscle-type receptors, found at the neuromuscular junction, receptors are either the embryonic form, composed of α1, β1, δ, and γ subunits in a 2:1:1:1 ratio, or the adult form composed of α1, β1, δ, and ε subunits in a 2:1:1:1 ratio. The neuronal subtypes are various combinations of twelve different nicotinic receptor subunits: α2 through α10 and β2 through β4. In both muscle-type and neuronal-type receptors, the subunits are somewhat similar to one another, especially in the hydrophobic regions | + | In vertebrates, nicotinic receptors are classified by their main sites of expression into two subtypes: muscle-type nicotinic receptors and neuronal-type nicotinic receptors. In the muscle-type receptors, found at the neuromuscular junction, receptors are either the '''embryonic''' form, composed of '''α1''', '''β1''', '''δ''', and '''γ''' subunits in a '''2:1:1:1''' ratio, or the '''adult''' form composed of '''α1''', '''β1''', '''δ''', and '''ε''' subunits in a '''2:1:1:1''' ratio. The neuronal subtypes are various combinations of twelve different nicotinic receptor subunits: '''α2''' through '''α10''' and '''β2''' through '''β4'''. In both muscle-type and neuronal-type receptors, the subunits are somewhat similar to one another, especially in the hydrophobic regions. |
</span> | </span> | ||
| + | |||
| + | |||
| + | |||
<span style="font-size:110%"> | <span style="font-size:110%"> | ||
| - | [[Image:Acetylcholine Binding.gif |left| | + | [[Image:Acetylcholine Binding.gif |left|350px|alt=text|Caption]] |
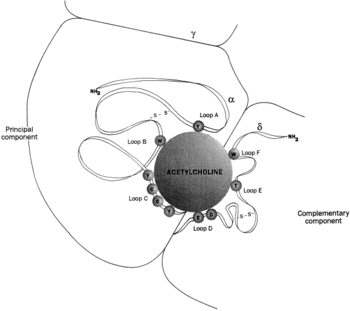
| + | As shown in the picture to the right, the two α-subunits are colored orange in this homeric receptor example. The two α-subunits each contain a ligand binding site colored red. '''Cys-192''' and '''Cys-193''', which are close proximity to the allosteric binding sites on the α-subunits for acetylcholine have been experimentally tested to show that disulfide residues provides stability in the binding of the neurotransmitter. The image (bottom left) shows a simplified depiction of the main components of one of the binding sites in a nAChR. Acetylcholine is shown to interact with various amino acid residues but specifically with the series of amino acids: Tyrosine (Y), Cysteine (C), Cysteine (C), and Tyrosine (Y). The cysteine sulfide residues form the disulfide interaction which aids in acetylcholine binding. The disulfide bonds facilitate the loop formations necessary for the appropriate bonds being formed with acetylcholine. | ||
</span> | </span> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
== '''Medical Applications''' == | == '''Medical Applications''' == | ||
| + | <span style="font-size:110%"> | ||
| + | |||
| + | </span> | ||
== '''References''' == | == '''References''' == | ||
Revision as of 06:29, 1 May 2012
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
ACETYLCHOLINE RECEPTOR
Nicotinic Acetylcholine Receptor (nAChRs) is a neurotransmitter receptor also known as a specialized integral membrane protein. Integral membrane proteins are permanently bound to the phospholipid bilayer. They provide a communication pathway between the internal and external environments of the cell. Acetylcholine receptor proteins reside as ligand-gated ion channels on the motor end plate of the postsynaptic cleft in the neuromuscular junction.
MechanismThe neurotransmitter acetylcholine binds specifically to the α-subunits in the homeric receptor example. StructureNicotinic Acetylcholine Receptor is composed up five distinct subunits (shown in figure to left): α1, α2, β1, δ, and ε at approximately a molecular mass of 290 kDa. The subunits are symmetrically arranged around a central pore. Each subunit is a transmembrane domain with the N-terminus and C-terminus located on the extracellular side of the plasma membrane. Each subunit are composed of long protein chains that extend across the cell membrane. The subunits have a composition of around ~20% beta sheets and ~40% alpha helices from about ~370 residues in total.
In vertebrates, nicotinic receptors are classified by their main sites of expression into two subtypes: muscle-type nicotinic receptors and neuronal-type nicotinic receptors. In the muscle-type receptors, found at the neuromuscular junction, receptors are either the embryonic form, composed of α1, β1, δ, and γ subunits in a 2:1:1:1 ratio, or the adult form composed of α1, β1, δ, and ε subunits in a 2:1:1:1 ratio. The neuronal subtypes are various combinations of twelve different nicotinic receptor subunits: α2 through α10 and β2 through β4. In both muscle-type and neuronal-type receptors, the subunits are somewhat similar to one another, especially in the hydrophobic regions.
As shown in the picture to the right, the two α-subunits are colored orange in this homeric receptor example. The two α-subunits each contain a ligand binding site colored red. Cys-192 and Cys-193, which are close proximity to the allosteric binding sites on the α-subunits for acetylcholine have been experimentally tested to show that disulfide residues provides stability in the binding of the neurotransmitter. The image (bottom left) shows a simplified depiction of the main components of one of the binding sites in a nAChR. Acetylcholine is shown to interact with various amino acid residues but specifically with the series of amino acids: Tyrosine (Y), Cysteine (C), Cysteine (C), and Tyrosine (Y). The cysteine sulfide residues form the disulfide interaction which aids in acetylcholine binding. The disulfide bonds facilitate the loop formations necessary for the appropriate bonds being formed with acetylcholine.
Medical Applications
References |