Human Immunodeficiency Virus-1 Protease
Introduction
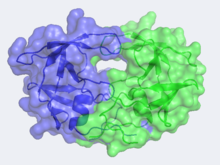
HIV -1 protease (HIV PR ) is a retroviral aspartyl protein that is derived from HIV-1, a lentivirus that is best characterized for its ability to lower host immunity by infecting CD4+ T lymphocytes, macrophages, and dendritic cells. Unlike most members of aspartyl protease class, which generally exist as two domain monomers, HIV protease is a dimmer with two identical subunits that are comprised of 99 amino acids. The HIV PR, together with single stranded RNA (ssRNA), reverse transcriptase, integrase, and other viral factors, is found inside the HIV-1 virion. As an important viral protein, it plays a crucial role in successful viral propagation.
Structure & Function
Structure of HIV-1 Protease
The X-ray structure of HIV-1 protease reveals that it is composed of two symmetrically related subunits, each consisting of 99 amino acid residues. The subunits come together in such as way as to form a tunnel where they meet. This tunnel is of critical importance because the active site of the protease is located in its interior. The active site consists of two Asp-Thr-Gly conserved sequences, making it a member of the aspartyl protease family. The two Asp's are essential catalytic residues that activate a water molecule to hydrolytically cleave the polyprotein that binds in the tunnel.[1] You may be wondering how a polyprotein makes its way into the active-site tunnel, as the tunnel appears to be too narrow to admit it. The key is the two flexible flaps on the top of the tunnel that move to allow proteins to enter the tunnel. The flaps undergo a dramatic movement, shifting from an open to a closed conformation to bind the target in an appropriate conformation for cleavage.
Mechanism
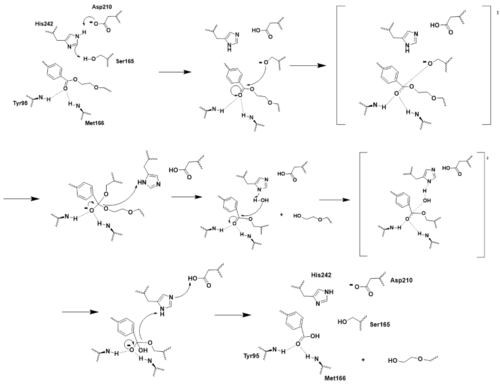 Mechanism for HIV-1 Protease
Mechanism for HIV-1 Protease
Applications & Research
When a HIV virus infects an organism it tends to multiply within the body’s cells. The virus is then released to infect other cells. In this manner, the infection of HIV infects the newly made cells of the body. While the viruses are produced, proteins and enzymes used to manufacture the DNA in addition to other components of the virus are made. In this case, protease is that enzyme that is needed to bring the structural and enzymes of the virus together. Protease drugs are what could inhibit this virus.
- Design of a HIV-1 Protease inhibitor - Free-energy parameterization of enzyme-inhibitor binding.
Based on the crystal structure data and protein receptor ligand complexes studied, interatomic interactions that work on burying atoms and find the statistical preference for amino acid pairs. A free energy model of the receptor-ligand is formulated and helps in showing the interfacial interactions. The interaction strength of this model has a reliability of ±1.5 kcal/mol, which reveals the importance of atomic interaction to stabilize the receptor-ligand interface. The analysis of a binding motif of HIV-1 protease inhibitor complex shows the important contacts instead of the set of atoms.
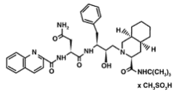
Saquinavir mesylate is a white to off-white, very fine powder with an aqueous solubility of 2.22 mg/mL at 25°C.
1) Saquinavir (Invirase) is known to be one of the first FDA approved protease inhibitor for HIV treatment. This usually occurs by HIV protease binding an active site tunnel tightly, which will prevent polyproteins from also binding. HIV’s chemical structure has the ability to mimic the tetrahedral intermediate of the hydrolytic reaction to interact strong with the catalytic Asp residues.
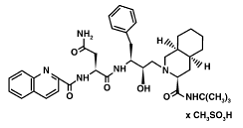
Saquinavir mesylate is a white to off-white, very fine powder with an aqueous solubility of 2.22 mg/mL at 25°C.
Knowing that, Saquinavir is an uncleavable ligand by studying its similar conformational changes in binding saquinavir or a polypeptide. ((
http://www.rxlist.com/invirase-drug.htm))))
2) Indinavir (Crixivan) this protease inhibitor is an oral medication that blocks the protease activity and leads to defect the viruses. Once these viruses are unable to reach the body’s cells, it will decrease in number
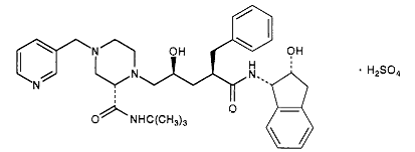
Ritonavir has a molecular weight of 711.88. It is very soluble in water and in methanol.
This inhibitor doesn’t have the ability to prevent the HIV transmission among individuals, nor does it cure AIDS or HIV infection. Indinavir is approved by the FDA in March 1995. ((((
http://www.medicinenet.com/indinavir/article.htm )))
3) Ritonavir (Norvir), has the same effect of indinavir on HIV protease. It was approved by the FDA in June 1999. This drug consists of capsules or tablets 100 mg; Solution: 80 mg/ml. It is recommended that adults consume 600 mg twice a day and then increase by 100 mg in every 2 days.
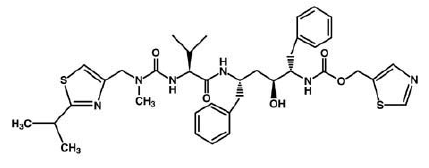
Ritonavir is soluble in isopropanol and practically insoluble in water.
Ritonavir can interact with many drugs leading those drugs to increase or decrease in effectiveness. It increases the concentration of Mycobutin and Viagra; however, decrease the concentration of Demerol. ((((
http://www.medicinenet.com/ritonavir/article.htm))))
4) Nelfinavir (Viracept), Nelfinavir was approved by the FDA in March, 1997. Nelfinavir has not been effectively evaluated in pregnant women. Viracept prevents T-cells that have been infected with HIV from producing new HIV.
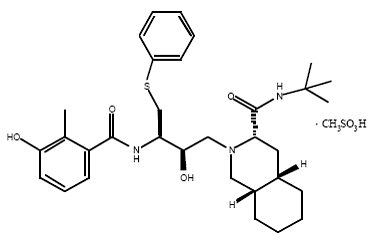
Nelfinavir is slightly soluble in water at pH ≤ 4 and completely soluble in methanol.
Usually given to patients through tablets. Each tablet contains inactive ingredients such as: calcium silicate, magnesium stearate, hypromellose, and triacetin. ( Picture )
http://www.rxlist.com/viracept-drug.htm
((http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001007/))
[1]
References
- ↑ Santos LO, Vitorio BS, Branquinha MH, Silva CM, Santos AL, d'Avila-Levy CM. Nelfinavir is effective in inhibiting the multiplication and aspartic peptidase activity of Leishmania species, including strains obtained from HIV-positive patients. J Antimicrob Chemother. 2012 Oct 28. PMID:23109184 doi:10.1093/jac/dks410