User:Erin May/Sandbox 1
From Proteopedia
(→PrP<sup>C</sup> natural monomer) |
(→PrP<sup>C</sup> natural monomer) |
||
| Line 22: | Line 22: | ||
The following residue alterations confer increased susceptibility to the unfolding of alpha helices characteristic of spongiform encephalopathies. | The following residue alterations confer increased susceptibility to the unfolding of alpha helices characteristic of spongiform encephalopathies. | ||
| - | Residue 129 Val-->Met confers increased susceptibility to the catalytic unfolding of the alpha helices characteristic of spongiform encephalopathies. As of January 2010, only individuals with homozygous | + | Residue 129 Val-->Met confers increased susceptibility to the catalytic unfolding of the alpha helices characteristic of spongiform encephalopathies. In monomeric form, <scene name='User:Erin_May/Sandbox_1/Methionine_129/1'>Methionine 129</scene>, does not offer a possible mechanism of unfolding or aggregation. As of January 2010, only individuals with homozygous Metionine129 had been diagnosed with infectious Creutzfeldt–Jakob disease. |
Residues R164 to S170 exhibit species specific residues. This region is most variable between species. Residue shifts in this section increase or decrease susceptibility from inter-species transmission of TSE's.<ref name="Lee">PMID:19927125</ref> | Residues R164 to S170 exhibit species specific residues. This region is most variable between species. Residue shifts in this section increase or decrease susceptibility from inter-species transmission of TSE's.<ref name="Lee">PMID:19927125</ref> | ||
Revision as of 08:48, 27 November 2012
Contents |
Prions as a disease causing agent
Prions are infectious or genetic misfolded proteins which act as templates upon which properly folded prion protein monomers can aggregate. Prions contain no nucleic acid such as other infectoius molecules or organisms. Human Prion Protein or Major Prion protein, exists as a normal constituent of human cells, found mostly in the brain[2] and is called PrPC.[3] PrPC is composed of mostly helix whereas the infectious form, PrPSc, is composed of high percentage beta sheets.[3]
The diseases prions confer are neurodegenerative disorders which result from the large scale aggregation of these proteins. For more information about the infections related to prions see Transmissible spongiform encephalopathy at Wikipedia.
Unfolding Mechanism
Currently, the mechanism by which a template prion unfolds a the helices of a properly folded prion protein is unknown. Specific residues have been shown to either confer resistance or lend themselves to this unfolding.
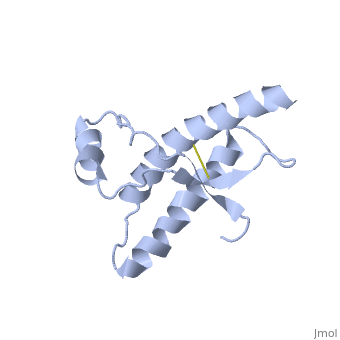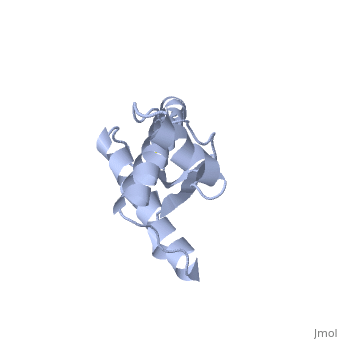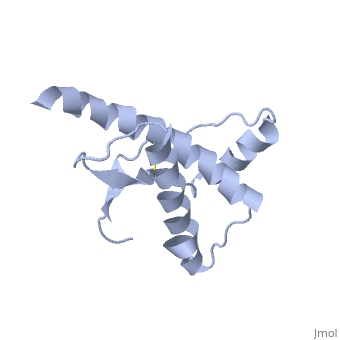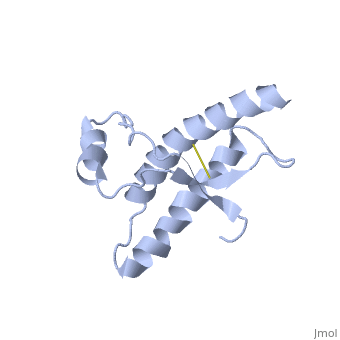
PrPC natural monomer
| |||||||||||
PrPSc
| |||||||||||
Dimer Form
| |||||||||||
Reference List
- ↑ Image of Creutzfeldt-Jakob positive brain tissue was obtained from The CDC's Public Health Image Library.
- ↑ Centers for Disease Control and Prevention
- ↑ 3.0 3.1 Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13363-83. PMID:9811807
- ↑ 4.0 4.1 Lee S, Antony L, Hartmann R, Knaus KJ, Surewicz K, Surewicz WK, Yee VC. Conformational diversity in prion protein variants influences intermolecular beta-sheet formation. EMBO J. 2010 Jan 6;29(1):251-62. Epub 2009 Nov 19. PMID:19927125 doi:10.1038/emboj.2009.333
- ↑ 5.0 5.1 5.2 Zhang Y, Swietnicki W, Zagorski MG, Surewicz WK, Sonnichsen FD. Solution structure of the E200K variant of human prion protein. Implications for the mechanism of pathogenesis in familial prion diseases. J Biol Chem. 2000 Oct 27;275(43):33650-4. PMID:10954699 doi:10.1074/jbc.C000483200
- ↑ 6.0 6.1 6.2 Knaus KJ, Morillas M, Swietnicki W, Malone M, Surewicz WK, Yee VC. Crystal structure of the human prion protein reveals a mechanism for oligomerization. Nat Struct Biol. 2001 Sep;8(9):770-4. PMID:11524679 doi:10.1038/nsb0901-770