Acetylcholinesterase
From Proteopedia
| Line 2: | Line 2: | ||
[[Image:small_wh_ray0001.gif|left|150px]]<br /> | [[Image:small_wh_ray0001.gif|left|150px]]<br /> | ||
{{STRUCTURE_1ea5| PDB=1ea5 | SIZE=500| SCENE=Acetylcholinesterase/New_down_gorge/7 |right|CAPTION=Acetylcholinesterase ([[1ea5]]) }} | {{STRUCTURE_1ea5| PDB=1ea5 | SIZE=500| SCENE=Acetylcholinesterase/New_down_gorge/7 |right|CAPTION=Acetylcholinesterase ([[1ea5]]) }} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
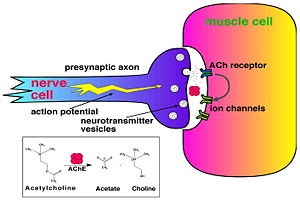
'''Acetylcholinesterase''' (AChE) is key enzyme in the nervous system of animals. By rapid hydrolysis of the neurotransmitter, [[Acetylcholine|acetylcholine]] (ACh), AChE terminates neurotransmission at cholinergic synapses. It is a very fast enzyme, especially for a serine hydrolase, functioning at a rate approaching that of a diffusion-controlled reaction. AChE inhibitors are among the key drugs approved by the FDA for management of Alzheimer's disease (AD). The powerful toxicity of organophosphorus (OP) poisons is attributed primarily to their potent AChE inhibitors. For more details see<br /> | '''Acetylcholinesterase''' (AChE) is key enzyme in the nervous system of animals. By rapid hydrolysis of the neurotransmitter, [[Acetylcholine|acetylcholine]] (ACh), AChE terminates neurotransmission at cholinergic synapses. It is a very fast enzyme, especially for a serine hydrolase, functioning at a rate approaching that of a diffusion-controlled reaction. AChE inhibitors are among the key drugs approved by the FDA for management of Alzheimer's disease (AD). The powerful toxicity of organophosphorus (OP) poisons is attributed primarily to their potent AChE inhibitors. For more details see<br /> | ||
*[[AChE bivalent inhibitors]] <br /> | *[[AChE bivalent inhibitors]] <br /> | ||
Revision as of 11:52, 20 March 2013
| |||||||||
| Acetylcholinesterase (1ea5) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Activity: | Acetylcholinesterase, with EC number 3.1.1.7 | ||||||||
| Related: | 1amn, 1ax9, 1cfj, 1dx6, 1e3q, 1e66, 1eea, 1eve, 1fss, 1oce, 1qid, 1qie, 1qif, 1qig, 1qih, 1qii, 1qij, 1qik, 1qim, 1qti, 1som, 1vot, 1vxo, 1vxr, 2ace, 2ack, 2dfp, 3ace | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Acetylcholinesterase (AChE) is key enzyme in the nervous system of animals. By rapid hydrolysis of the neurotransmitter, acetylcholine (ACh), AChE terminates neurotransmission at cholinergic synapses. It is a very fast enzyme, especially for a serine hydrolase, functioning at a rate approaching that of a diffusion-controlled reaction. AChE inhibitors are among the key drugs approved by the FDA for management of Alzheimer's disease (AD). The powerful toxicity of organophosphorus (OP) poisons is attributed primarily to their potent AChE inhibitors. For more details see
- AChE bivalent inhibitors
- Torpedo californica acetylcholinesterase with bifunctional inhibitor
- Acetylcholinesterase: Substrate Traffic and Inhibition
- Acetylcholinesterase Inhibitor Pharmacokinetics
- Acetylcholinesterase inhibitors
- AChE inhibitors and substrates
- Human Acetylcholinesterase
- Acetylcholinesterase inhibited by nerve agent soman
- Acetylcholinesterase with DFP
- Acetylcholinesterase with OTMA
- Acetylcholinesterase with acetylcholine
- Complex of TcAChE with an iminium galanthamine derivative
- Complex of TcAChE with bis-acting galanthamine derivative
- Cholinesterase
- Huperzine A Complexed with Acetylcholinesterase
- Torpedo Californica Acetylcholinesterase in complex with an (R)-Tacrine-(10)-Hupyridone inhibitor
- Torpedo Californica Acetylcholinesterase in complex with an (S)-Tacrine-(10)-Hupyridone inhibitor
- Torpedo californica acetylcholinesterase with alkylene-linked tacrine dimer (5 carbon linker)
- Torpedo californica acetylcholinesterase with alkylene-linked tacrine dimer (7 carbon linker)
- Torpedo californica acetylcholinesterase with bifunctional inhibitor
- Tetramerization domain of acetylcholinesterase
See also a model of African Malaria Mosquito Acetylcholinesterase.
| |||||||||||
3D Structures of AChE
Updated on 20-March-2013
Acetylcholinesterase - AChE native
3lii, 4ey4 – hAChE - recombinant human
1ea5, 2ace – TcAChE – trigonal – Torpedo californica
2j3d – TcAChE – monoclinic
1w75 – TcAChE – orthorhombic
2vt6, 2vt7 – TcAChE – different dosage
1qid to 1qim - TcAChE synchrotron radiation damage
1j06, 1maa – mAChE - mouse
1qo9 – DmAChE - Drosophila
1eea, 1c2b – electrophorus AChE – Electric eel - Orthorhombic
1c2o – electrophorus AChE – Electric eel - Monoclinic
AChE inhibitors (In Different Languages)
1eve AChE-Aricept complex, 1eve (Arabic), 1eve (Chinese), 1eve (Italian), 1eve (Russian), 1eve (Spanish), 1eve (Turkish)
1vot AChE-Huperzine A complex, 1vot (Chinese)
AChE active site inhibitors conjugating at the bottom of the active site gorge
2w9i – TcAChE + methylene blue
2wls – MosAChE + AMTS13
2vq6 – TcAChE + 2-PAM
2j3q – TcAChE + Thioflavin T
2ha0 – mAChE + ketoamyltrimethylammonium
2h9y – mAChE + TMTFA
1gpk, 1gpn, 1vot – TcAChE + huperzine
4ey5 – hAChE + huperzine
1gqr – TcAChE + rivastigmine
1gqs – TcAChE + NAP
1e66 – TcAChE + huprine
4a16 – mAChE + huprine
1dx4, 1qon – DmAChE + tacrine derivative
1oce – TcAChE + MF268
1ax9, 1ack – TcAChE + edrophonium
1amn – TcAChE + TMTFA
1acj – TcAChE + tacrine
1u65 – TcAChE + CPT-11
2bag - TcAChE + ganstigmine
2xi4 - TcAChE + aflatoxin
4ara, 4arb - mAChE + inhibitor
AChE peripheral site inhibitors conjugating at the surface of the protein
1ku6, 1mah - mAChE + fasciculin 2
1j07 - mAChE + decidium
1n5m - mAChE + gallamine
1n5r - mAChE + propidium
1b41, 1f8u - hAChE + fasciculin 2
1fss - TcAChE + fasciculin 2
2x8b - hAChE + fasciculin 2 + tabun
AChE bis inhibitors spanning the active site gorge
3i6m – TcAChE + N-piperidinopropyl galanthamine
3i6z - TcAChE + saccharinohexyl galanthamine
1zgb, 1zgc – TcAChE + tacrine (10) hupyridone
2w6c – TcAChE + bis-(-)-nor-meptazinol
2ckm, 2cmf – TcAChE + bis-tacrine
2cek – TcAChE + N-[8-(1,2,3,4-tetrahydroacridin-9-ylthio)octyl]-1,2,3,4-tetrahydroacridin-9-amine
1ut6 - TcAChE + N-9-(1,2,3,4-tetrahydroacridinyl)-1,8-diaminooctane
1odc - TcAChE + N-4-quinolyl-N-9-(1,2,3,4-tetrahydroacridinyl)-1,8-diaminooctane
1w4l, 1w6r, 1w76, 1dx6, 1qti - TcAChE + galanthamine and derivative
4ey6 - hAChE + galanthamine
4ey7 - hAChE + donepezil
1q83, 1q84 - mAChE + TZ2PA6
1h22, 1h23 – TcAChE + bis-hupyridone
1hbj – TcAChE + quinoline derivativev
1e3q – TcAChE + bw284c51
1eve – TcAChE + e2020
1acl – TcAChE + decamethonium
3zv7 - TcAChE + bisnorcymserine
AChE organophosphate inhibitors causing irreversible inhibition
2wu3 – mAChE + fenamiphos and HI-6
2wu4 – mAChE + fenamiphos and ortho-7
2jgf - mAChE + fenamiphos
2wfz, 2wg0, 2wg2, 1som - TcAChE + soman
2wg1 - TcAChE + soman + 2-PAM
2whp, 2whq, 2whr – mAChE + sarin and HI-6
2jgg - mAChE + sarin
2jgl - mAChE + VX and sarin
1cfj - TcAChE + sarin, GB
3dl4, 3dl7 – mAChE + tabun
2jey – mAChE + HLO-7
2c0p, 2c0q - mAChE + tabun
2jez - mAChE + tabun + HLO-7
2jf0 - mAChE + tabun + Ortho-7
2jgh - mAChE + VX
1vxo, 1vxr - TcAChE + VX
2jgi, 2jgm - mAChE + DFP
1dfp - TcAChE + DFP
2jgj, 2jgk, 2jge - mAChE + methamidophos
2gyu - mAChE + HI-6
2gyv - mAChE + Ortho-7
2gyw - mAChE + obidoxime
3gel - TcAChE + methyl paraoxon
AChE substrate analogues mimicking the binding of the substrate acetylcholine
2ha4 – mAChE (mutant) + acetylcholine
2vja, 2vjb, 2vjc, 2vjd, 2cf5 – TcAChE + 4-oxo-N,N,N-trimethylpentanaminium
2v96, 2v97, 2v98, 2v99 – TcAChE + 1-(2-nitrophenyl)-2,2,2-trifluoroethyl-arsenocholine
2ha2 – mAChE + succinylcholine
2ha3 - mAChE + choline
2ha5 – mAChE (mutant) + acetylthiocholine
2ha6 – mAChE (mutant) + succinylthiocholine
2ha7 – mAChE (mutant) + butyrylthiocholine
2ch4, 2c58 – TcAChE + acetylthiocholine
2c5g – TcAChE + thiocholine
2va9 - TcAChE + ‘caged’ arsenocholine
Others...
2j4f – TcAChE + Hg
1vzj – TcAChE tetramerization domain
1jjb – TcAChE + PEG
1qie, 1qif, 1qig, 1qih, 1qii, 1qij, 1qik – TcAChE synchrotron radiation damage
3m3d – TcAChE + Xe
Additional Resources
For additional information, see:
Alzheimer's Disease
AChE inhibitors and substrates
AChE inhibitors and substrates (Part II)
AChE inhibitors and substrates (Part III)
AChE bivalent inhibitors
AChE bivalent inhibitors (Part II)
External Links
- Acetylcholinesterase Tutorial by Karl Oberholser, Messiah College
- PDB Molecule of the Month - Acetylcholinesterase
- Movies: X-ray Damage in ACh & Nature's Vacuum Cleaner by R. Gillilan, Cornell Univ
References
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Botti SA, Felder CE, Lifson S, Sussman JL, Silman I. A modular treatment of molecular traffic through the active site of cholinesterase. Biophys J. 1999 Nov;77(5):2430-50. PMID:10545346
- ↑ 3.0 3.1 Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Biol. 1997 Jan;4(1):57-63. PMID:8989325
- ↑ Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3 A resolution. FEBS Lett. 1999 Dec 17;463(3):321-6. PMID:10606746
- ↑ Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9031-5. PMID:8415649
- ↑ Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry. 2002 Mar 19;41(11):3555-64. PMID:11888271
- ↑ Haviv H, Wong DM, Greenblatt HM, Carlier PR, Pang YP, Silman I, Sussman JL. Crystal packing mediates enantioselective ligand recognition at the peripheral site of acetylcholinesterase. J Am Chem Soc. 2005 Aug 10;127(31):11029-36. PMID:16076210 doi:http://dx.doi.org/10.1021/ja051765f
- ↑ Ravelli RB, Raves ML, Ren Z, Bourgeois D, Roth M, Kroon J, Silman I, Sussman JL. Static Laue diffraction studies on acetylcholinesterase. Acta Crystallogr D Biol Crystallogr. 1998 Nov 1;54(Pt 6 Pt 2):1359-66. PMID:10089512
- ↑ Harel M, Sonoda LK, Silman I, Sussman JL, Rosenberry TL. Crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site. J Am Chem Soc. 2008 Jun 25;130(25):7856-61. Epub 2008 May 31. PMID:18512913 doi:http://dx.doi.org/10.1021/ja7109822
- ↑ Greenblatt HM, Guillou C, Guenard D, Argaman A, Botti S, Badet B, Thal C, Silman I, Sussman JL. The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: implications for structure-based drug design. J Am Chem Soc. 2004 Dec 1;126(47):15405-11. PMID:15563167 doi:http://dx.doi.org/10.1021/ja0466154
- ↑ Kryger G, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure. 1999 Mar 15;7(3):297-307. PMID:10368299
- ↑ Sanson B, Nachon F, Colletier JP, Froment MT, Toker L, Greenblatt HM, Sussman JL, Ashani Y, Masson P, Silman I, Weik M. Crystallographic Snapshots of Nonaged and Aged Conjugates of Soman with Acetylcholinesterase, and of a Ternary Complex of the Aged Conjugate with Pralidoxime (dagger). J Med Chem. 2009 Jul 30. PMID:19642642 doi:10.1021/jm900433t
Treatments:AChE Inhibitor References
Treatments:Alzheimer's Disease
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, David Canner, Eran Hodis, Clifford Felder, Jaime Prilusky, Harry Greenblatt, Yechun Xu