We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Interferon
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| + | <StructureSection load='2hym' size='500' side='right' caption='Complex of the α-helical interferon α-2 with soluble IFN α/β receptor. Click on the green links to the left to view the structural aspects of interferons. PDB ID: [[2hym]])' scene='Interferons/Interferonaandreceptor/2'> | ||
'''Interferons''' were the first cytokines discovered and were identified by Isaacs and Lindenmann. These proteins were classified as interferons because they interfered with virus growth.<ref name="Isaacs" /> The initial experiments performed poorly characterized the interferons, and was based merely on bioactivity. Advances in scientific instrumentation and technique have allowed for greater understanding and visualization of not only the structure but also the mechanisms of the various types of inteferons.<ref name="Structure">PMID:2413490</ref> The interferons were originally classified as leukocyte (interferon-α), fibroblast (interferon-β), and immmune (interferon-γ), although today they are classified into types I (α, β, ε, κ, ω), II (γ), and III (λ).<ref name="Isaacs" /><ref name="Structure" /> | '''Interferons''' were the first cytokines discovered and were identified by Isaacs and Lindenmann. These proteins were classified as interferons because they interfered with virus growth.<ref name="Isaacs" /> The initial experiments performed poorly characterized the interferons, and was based merely on bioactivity. Advances in scientific instrumentation and technique have allowed for greater understanding and visualization of not only the structure but also the mechanisms of the various types of inteferons.<ref name="Structure">PMID:2413490</ref> The interferons were originally classified as leukocyte (interferon-α), fibroblast (interferon-β), and immmune (interferon-γ), although today they are classified into types I (α, β, ε, κ, ω), II (γ), and III (λ).<ref name="Isaacs" /><ref name="Structure" /> | ||
| - | |||
{{Clear}} | {{Clear}} | ||
| - | |||
| - | <StructureSection load='2hym' size='500' side='right' caption='Complex of the α-helical interferon α-2 with soluble IFN α/β receptor. Click on the green links to the left to view the structural aspects of interferons. PDB ID: [[2hym]])' scene='Interferons/Interferonaandreceptor/2'> | ||
==Type I== | ==Type I== | ||
Type I interferons are homologous helical cytokines that effect a wide variety of cells pleiotropically. These effects range from antiviral activity to antibacterial, antiprozoal, immunodulatory, and cell growth regulatory functions. Without Type I interferons, the survival of the higher vertebrates would be impossible. Because of their strong antiviral and antiproliferative effects, these interferons are used in the treatment of numerous cancers, hepatitis C, and multiple sclerosis. | Type I interferons are homologous helical cytokines that effect a wide variety of cells pleiotropically. These effects range from antiviral activity to antibacterial, antiprozoal, immunodulatory, and cell growth regulatory functions. Without Type I interferons, the survival of the higher vertebrates would be impossible. Because of their strong antiviral and antiproliferative effects, these interferons are used in the treatment of numerous cancers, hepatitis C, and multiple sclerosis. | ||
| Line 20: | Line 18: | ||
__NOTOC__ | __NOTOC__ | ||
| - | </StructureSection> | ||
| - | |||
== Comparison of three interferons == | == Comparison of three interferons == | ||
| + | *<scene name='Multiple_sclerosis/Ifnb_antiparallel_ce/3'>Interferon Alpha</scene> | ||
| + | *<scene name='Multiple_sclerosis/Ifnb_antiparallel_ce/3'>Interferon Beta</scene> | ||
| + | *<scene name='Multiple_sclerosis/Ifnb_antiparallel_ce/3'>Interferon Gamma</scene> | ||
{| | {| | ||
|<applet load='1ITF.pdb' name='A' size='300' frame='true' align='right' caption='Interferon Alpha' align='left' scene='Interferon/Ifn_alpha/5'/> | |<applet load='1ITF.pdb' name='A' size='300' frame='true' align='right' caption='Interferon Alpha' align='left' scene='Interferon/Ifn_alpha/5'/> | ||
| Line 30: | Line 29: | ||
|} | |} | ||
<center> | <center> | ||
| - | |||
| - | '''Synchronize the three applets showing interferons alpha, beta, and gamma by clicking the checkbox''' | ||
| - | <jmol> | ||
| - | <jmolCheckbox> | ||
| - | <target>A</target> | ||
| - | <!--<scriptWhenChecked>set syncMouse ON;set syncScript OFF;sync jmolAppletB,jmolAppletZ; sync > "set syncMouse | ||
| - | ON;set syncScript OFF"</scriptWhenChecked>--> | ||
| - | <scriptWhenChecked> sync jmolAppletB,jmolAppletZ </scriptWhenChecked> | ||
| - | <scriptWhenUnchecked> sync OFF</scriptWhenUnchecked> | ||
| - | <text> Synchronize</text> | ||
| - | </jmolCheckbox> | ||
| - | </jmol> | ||
| - | </center> | ||
| - | |||
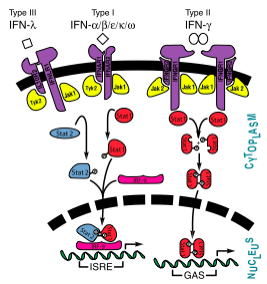
[[Image:InterferonSignalingPathway.png|600px|right|thumb|Interferon JAK-STAT Pathway showing interferons types I, II, and III<ref name="Isaacs">[http://www.jbc.org/content/282/28/20045.full?sid=cbf08059-44d4-4957-8ea7-0351cab9c2ac] Samuel, C.E. "Interferons, Interferon Receptors, Signal Transducer and Transcriptional Activators, and Inteferon Regulatory Factors." ''J Biol Chem'' 2007 282: 20045-20046. First Published on May 14, 2007, doi:10.1074/jbc.R700025200</ref>]] | [[Image:InterferonSignalingPathway.png|600px|right|thumb|Interferon JAK-STAT Pathway showing interferons types I, II, and III<ref name="Isaacs">[http://www.jbc.org/content/282/28/20045.full?sid=cbf08059-44d4-4957-8ea7-0351cab9c2ac] Samuel, C.E. "Interferons, Interferon Receptors, Signal Transducer and Transcriptional Activators, and Inteferon Regulatory Factors." ''J Biol Chem'' 2007 282: 20045-20046. First Published on May 14, 2007, doi:10.1074/jbc.R700025200</ref>]] | ||
| Line 55: | Line 40: | ||
Interferon-α <scene name='Multiple_sclerosis/Ifnawithreceptorcolored/1'>binds</scene> to an interferon receptor mainly with helices C and G. There are many <scene name='Multiple_sclerosis/Ifnawithreceptorintrxns/2'>residues</scene> within 4 angstroms of one another. These residues could form many <scene name='Multiple_sclerosis/Ifnawithreceptorintrxns/5'>different types of bonds</scene>, illustrated in white dotted lines. Given that interferon-α does not undergo many structural changes upon binding to interferon receptor II, Quadt-Akabayov et al. have concluded that the binding mechanism is similar to that of a lock and key. Interferons -α and -β interact with a receptor at the cell surface.<ref>[http://www.jbc.org/content/282/28/20045.full?sid=cbf08059-44d4-4957-8ea7-0351cab9c2ac] Samuel, C.E. "Interferons, Interferon Receptors, Signal Transducer and Transcriptional Activators, and Inteferon Regulatory Factors." ''J Biol Chem'' 2007 282: 20045-20046. First Published on May 14, 2007, doi:10.1074/jbc.R700025200</ref> This receptor has <scene name='Multiple_sclerosis/Ifnr_domains_labeled/1'>three domains</scene>: an | Interferon-α <scene name='Multiple_sclerosis/Ifnawithreceptorcolored/1'>binds</scene> to an interferon receptor mainly with helices C and G. There are many <scene name='Multiple_sclerosis/Ifnawithreceptorintrxns/2'>residues</scene> within 4 angstroms of one another. These residues could form many <scene name='Multiple_sclerosis/Ifnawithreceptorintrxns/5'>different types of bonds</scene>, illustrated in white dotted lines. Given that interferon-α does not undergo many structural changes upon binding to interferon receptor II, Quadt-Akabayov et al. have concluded that the binding mechanism is similar to that of a lock and key. Interferons -α and -β interact with a receptor at the cell surface.<ref>[http://www.jbc.org/content/282/28/20045.full?sid=cbf08059-44d4-4957-8ea7-0351cab9c2ac] Samuel, C.E. "Interferons, Interferon Receptors, Signal Transducer and Transcriptional Activators, and Inteferon Regulatory Factors." ''J Biol Chem'' 2007 282: 20045-20046. First Published on May 14, 2007, doi:10.1074/jbc.R700025200</ref> This receptor has <scene name='Multiple_sclerosis/Ifnr_domains_labeled/1'>three domains</scene>: an | ||
<scene name='Multiple_sclerosis/Ifnr_n_domain_labeled/1'>N-domain</scene>, with two disulfide bonds, a <scene name='Multiple_sclerosis/Ifnr_c_domain_labeled/1'>C-domain</scene>, with one disulfide bond, and a <scene name='Multiple_sclerosis/Ifnr_linker_region_labeled/1'>linker region</scene>. The <scene name='Multiple_sclerosis/Ifnr_termini_labeled/1'>termini regions</scene> of the receptor have no secondary structure, allowing for some serious flexibility, leading to <scene name='Multiple_sclerosis/Ifnr_clash_n-c/1'>eight clashes amongst the domains</scene>.<ref name="Interferon Receptor Structure">PMID:12842042</ref> | <scene name='Multiple_sclerosis/Ifnr_n_domain_labeled/1'>N-domain</scene>, with two disulfide bonds, a <scene name='Multiple_sclerosis/Ifnr_c_domain_labeled/1'>C-domain</scene>, with one disulfide bond, and a <scene name='Multiple_sclerosis/Ifnr_linker_region_labeled/1'>linker region</scene>. The <scene name='Multiple_sclerosis/Ifnr_termini_labeled/1'>termini regions</scene> of the receptor have no secondary structure, allowing for some serious flexibility, leading to <scene name='Multiple_sclerosis/Ifnr_clash_n-c/1'>eight clashes amongst the domains</scene>.<ref name="Interferon Receptor Structure">PMID:12842042</ref> | ||
| - | + | </StructureSection> | |
==References== | ==References== | ||
Revision as of 08:22, 8 May 2013
| |||||||||||
References
- ↑ 1.0 1.1 1.2 [1] Samuel, C.E. "Interferons, Interferon Receptors, Signal Transducer and Transcriptional Activators, and Inteferon Regulatory Factors." J Biol Chem 2007 282: 20045-20046. First Published on May 14, 2007, doi:10.1074/jbc.R700025200
- ↑ 2.0 2.1 Langer JA, Pestka S. Structure of interferons. Pharmacol Ther. 1985;27(3):371-401. PMID:2413490
- ↑ Quadt-Akabayov SR, Chill JH, Levy R, Kessler N, Anglister J. Determination of the human type I interferon receptor binding site on human interferon-alpha2 by cross saturation and an NMR-based model of the complex. Protein Sci. 2006 Nov;15(11):2656-68. Epub 2006 Sep 25. PMID:17001036 doi:10.1110/ps.062283006
- ↑ Voet, D., Voet, J.G., and C. Pratt. Fundamentals of Biochemistry 3rd Edition. Hoboken, NJ: John Wiley and Sons, 2008. Print.
- ↑ Kudo M. Management of hepatocellular carcinoma: from prevention to molecular targeted therapy. Oncology. 2010 Jul;78 Suppl 1:1-6. Epub 2010 Jul 8. PMID:20616576 doi:10.1159/000315222
- ↑ http://www.uniprot.org/uniprot/P00784
- ↑ Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012 Apr 2;122(4):1180-8. doi: 10.1172/JCI58649. Epub 2012 Apr 2. PMID:22466660 doi:10.1172/JCI58649
- ↑ Loma I, Heyman R. Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol. 2011 Sep;9(3):409-16. PMID:22379455 doi:10.2174/157015911796557911
- ↑ Quadt-Akabayov SR, Chill JH, Levy R, Kessler N, Anglister J. Determination of the human type I interferon receptor binding site on human interferon-alpha2 by cross saturation and an NMR-based model of the complex. Protein Sci. 2006 Nov;15(11):2656-68. Epub 2006 Sep 25. PMID:17001036 doi:10.1110/ps.062283006
- ↑ [2] Samuel, C.E. "Interferons, Interferon Receptors, Signal Transducer and Transcriptional Activators, and Inteferon Regulatory Factors." J Biol Chem 2007 282: 20045-20046. First Published on May 14, 2007, doi:10.1074/jbc.R700025200
- ↑ Chill JH, Quadt SR, Levy R, Schreiber G, Anglister J. The human type I interferon receptor: NMR structure reveals the molecular basis of ligand binding. Structure. 2003 Jul;11(7):791-802. PMID:12842042
3D Structures of interferon
Interferon-α
1itf - hIF 2A – NMR
2hym, 2kz1, 2lag, 3s9d - hIF 2A + IFR α/β
2rh2 – hIF 2B
3se3 - hIF 2B + IFR 1 + IFR 2
3oq3 - hIF 5 + IFR α/β
Interferon-β
1ifa, 1wu3 – IF – mouse
1au1 - hIF
Interferon-γ
1hig – hIF – human
1eku – hIF (mutant)
2rig – IF – rabbit
1rfb, 1d9c – IF – bovine
1fg9, 1fyh, 3bes – hIF + IFR α chain
Interferon-λ
3og4, 3og6 – hIF 1 + IFR
3hhc – hIF 4
Interferon-τ
1b5l – IF
Interferon-ω
3se4 - hIF 1 + IFR 1 + IFR 2
3piv – ZfIF 1 – Zebra fish
3piw - ZfIF 2
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Kirsten Eldredge, Alexander Berchansky, Joel L. Sussman, Karl Oberholser, Jaime Prilusky