Epidermal Growth Factor Receptor
From Proteopedia
| Line 1: | Line 1: | ||
| - | [[Image:3i2t1.png|left|200px|thumb|Crystal Structure of unliganded Drosophila Epidermal Growth Factor Receptor ectodomain, [[3i2t]]]]{{ | + | <StructureSection load='3i2t' size='450' side='right' scene='Epidermal_Growth_Factor_Receptor/Cv/1' caption=''> |
| - | + | [[Image:3i2t1.png|left|200px|thumb|Crystal Structure of unliganded Drosophila Epidermal Growth Factor Receptor ectodomain, [[3i2t]]]] | |
| - | + | {{Clear}} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
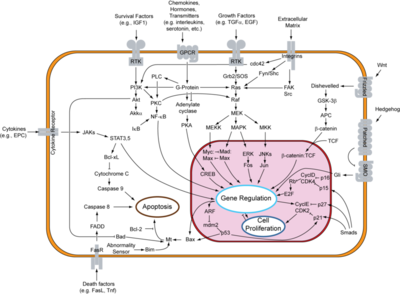
[[Epidermal Growth Factor Receptor]] (EGFR or ERBB1) is found on the cell surface and associates to homodimers upon binding of its ligands such as the Epidermal Growth Factor (EGF) to its extracellular domain. The dimerization stimulates autophosphorylation of several tyrosine residues in the intracellular kinase domain which signal downstream transduction cascades. A human EGFR-2 (HER-2 or ERBB2) is involved in breast [[Cancer|cancer]] and is a major target for breast cancer [[Pharmaceutical Drugs|therapeutics]]. ERBB3 uses neuregulin as a ligand. ERBB4 is a closely related receptor tyrosine kinase. The images at the left and at the right correspond to one representative EGFR, ''i.e.'' crystal structure of unliganded Drosophila Epidermal Growth Factor Receptor ectodomain ([[3i2t]]). | [[Epidermal Growth Factor Receptor]] (EGFR or ERBB1) is found on the cell surface and associates to homodimers upon binding of its ligands such as the Epidermal Growth Factor (EGF) to its extracellular domain. The dimerization stimulates autophosphorylation of several tyrosine residues in the intracellular kinase domain which signal downstream transduction cascades. A human EGFR-2 (HER-2 or ERBB2) is involved in breast [[Cancer|cancer]] and is a major target for breast cancer [[Pharmaceutical Drugs|therapeutics]]. ERBB3 uses neuregulin as a ligand. ERBB4 is a closely related receptor tyrosine kinase. The images at the left and at the right correspond to one representative EGFR, ''i.e.'' crystal structure of unliganded Drosophila Epidermal Growth Factor Receptor ectodomain ([[3i2t]]). | ||
| Line 29: | Line 10: | ||
== '''EGFR and Lung Cancers of "Never Smokers"''' == | == '''EGFR and Lung Cancers of "Never Smokers"''' == | ||
| - | + | ||
Tyrosine kinases in general control critical cellular activities through regulation of signal pathways (3). This regulation is essential for controlling the cell and making sure it acts normally and goes through the normal cell cycle. However, when these kinases are mutated, they can lead to cancer as a result (3). Mutations in EGFR as a result can also lead to cancers, and this is why understanding how EGFR works is an essential aspect for the treatment of certain cancers. In cancer, cells divide rapidly without control and an uncontrolled or unregulated EGF receptor will and can result in uncontrolled cell division. Therefore, inhibition of EGFR can be an essential part of controlling cancers caused by mutations in EGFR. | Tyrosine kinases in general control critical cellular activities through regulation of signal pathways (3). This regulation is essential for controlling the cell and making sure it acts normally and goes through the normal cell cycle. However, when these kinases are mutated, they can lead to cancer as a result (3). Mutations in EGFR as a result can also lead to cancers, and this is why understanding how EGFR works is an essential aspect for the treatment of certain cancers. In cancer, cells divide rapidly without control and an uncontrolled or unregulated EGF receptor will and can result in uncontrolled cell division. Therefore, inhibition of EGFR can be an essential part of controlling cancers caused by mutations in EGFR. | ||
| Line 37: | Line 18: | ||
Ten percent of lung cancers occur in patients who are deemed to be "never smokers" which are people who have smoked less than 100 cigarettes in lifetime (3). Therefore, a large number of people are affected by lung cancer without smoking, and this is quite important. What do these people have in common if they are not smoking cigarettes? A study showed that 75% of cancers with a mutation in EGFR were from these "never smokers" (3). This means that gefitinib and erlotinib are most likely going to be effective on people who have not smoked because there is a high correlation of them having these mutations in EGFR. Studying these mutations and what causes them could be the next step in understanding how to prevent these types of lung cancers. | Ten percent of lung cancers occur in patients who are deemed to be "never smokers" which are people who have smoked less than 100 cigarettes in lifetime (3). Therefore, a large number of people are affected by lung cancer without smoking, and this is quite important. What do these people have in common if they are not smoking cigarettes? A study showed that 75% of cancers with a mutation in EGFR were from these "never smokers" (3). This means that gefitinib and erlotinib are most likely going to be effective on people who have not smoked because there is a high correlation of them having these mutations in EGFR. Studying these mutations and what causes them could be the next step in understanding how to prevent these types of lung cancers. | ||
| + | </StructureSection> | ||
| + | __NOTOC__ | ||
== 3D Structures of Epidermal Growth Factor Receptor == | == 3D Structures of Epidermal Growth Factor Receptor == | ||
Revision as of 11:36, 26 August 2013
| |||||||||||
3D Structures of Epidermal Growth Factor Receptor
Updated on 26-August-2013
EGFR extracellular domain
3ltf – DmEGFR extracellular domain+spitz protein – Drosophila melanogaster
3i2t - DmEGFR extracellular domain
3n85 – hERBB2 extracellular domain+FAB37 light and heavy chains – human
1yy9 – hERBB1 extracellular domain+antibody
1m6b – hERBB3 extracellular domain
1mox - hEGFR extracellular domain+TGF alpha
1ivo, 1nql, 3njp - hEGFR extracellular domain+hEGF
3qwq – hEGFR extracellular domain + adnectin
3ltg - EGFR extracellular domain+ Spitz (mutant) – Drosophila melanogaster
EGFR transmembrane domain
2ks1 – hERBB1+hERBB2 transmembrane domains
EGFR kinase domain
3gt8 – hEGFR kinase domain+peptide+AMPPNP
2qs2 – hERBB1 kinase domain
2gs6 - hERBB1 kinase domain+peptide
2gs7 - hERBB1 kinase domain+AMPPNP
2eb2, 2jit, 1m14, 4i1z, 4i20 – hERBB1 kinase domain (mutant)
2jiu, 2jiv, 2ito, 2itp, 2itq, 2itt, 2itu, 2itw, 2itz, 2j6m, 2j5e, 2j5f, 1m17, 3w2o, 3w2p, 3w2q, 3w2r 3w2s - hERBB1 kinase domain (mutant)+inhibitor
3ug2, 2ity, 4g5p, 4hjo, 4i22, 4i24 - hERBB1 kinase domain (mutant) + cancer drug
4g5j, 4i23 - hERBB1 kinase domain + cancer drug
2rf9, 2rfd, 2rfe, 1xkk, 3lzb, 3poz, 3w32, 3w33 - hERBB1 kinase domain+inhibitor
2eb3, 2itn, 2itv, 2itx, 3vjn - hERBB1 kinase domain (mutant)+AMPPNP
3vjo - hERBB1 kinase domain (mutant)+AMPPNP
3b2u - hERBB1 kinase domain+IMC-11F8 FAB fragment
3p0y - hERBB1 kinase domain+ FAB
4i21 - hERBB1 kinase domain + ERBB receptor feedback inhibitor 1
3kex – hERBB3 kinase domain
EGFR juxtamembrane domain
3gop – hERBB1 kinase and juxtamembrane domains
1z9i - hERBB1 juxtamembrane domain
2m20 – hERBB1 transmembrane and juxtamembrane domains (mutant) - NMR
EGFR fragments
3i7z – hEGFR fragment+hTyrosine phosphatase
3g5v, 3g5y, 3g5z, 3g5x – EGFR fragment+antibody – mouse
3ika – hERBB1 (mutant)+inhibitor
2rgp – hERBB1+hydrazone
3bel – hERBB1+oxime
3c09 – hERBB1 domain III+antibody
2b2u, 3b2v – hERBB1 fragment+antibody
3buo – EGFR 13-mer+hCBL N-terminal
Additional Resources
For additional information, see: Cancer
References
1.Sherrill, Jennifer M., and Jack Kyte. "Activation of Epidermal Growth Factor Receptor by Epidermal Growth Factor†." Biochemistry 35 (1996): 5705-718. Print.
2.Herbst, R. S. "Review of epidermal growth factor receptor biology." Int J Radiat Oncol Biol Phys. 59 (2994). Print.
3.Pao, William, and Vincent Miller. "EGF receptor gene mutations are common in lung cancers from ‘‘never smokers’’ and are associated with sensitivity of tumors to gefitinib and erlotinib." PNAS 101 (2004). Print.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky