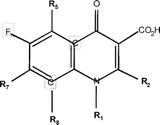
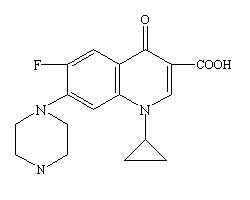
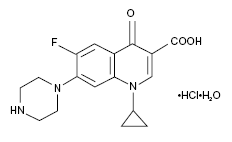
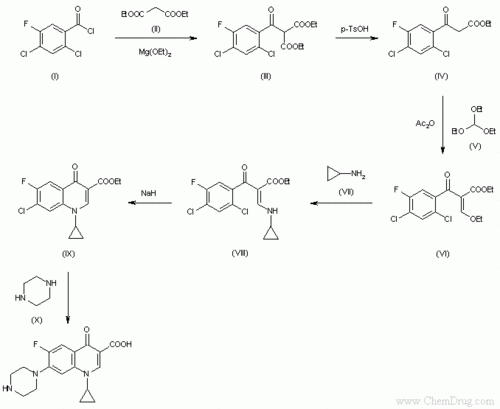
Ciprofloxacin
From Proteopedia
(Difference between revisions)
| Line 77: | Line 77: | ||
=== Efflux Pump Interaction === | === Efflux Pump Interaction === | ||
| - | Certain bacteria (''Escherichia coli'', for example) contain a proton motive force-dependent multidrug <scene name='Ciprofloxacin/Cv/1'>efflux pump</scene>, which, as the name suggests, grants the bacteria resistance to certain drugs <ref>Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. ''Journal of Bacteriology, 188(20)'', 7290-7296. </ref>. In ''Escherichia coli'', the efflux system that confers particular drug resistance is a tripartite transmembrane resistance structure known as "AcrAB-TolC" <ref>Husain, F., Nikaido, H. (2010). Substrate path in the AcrB multidrug efflux pump of Escherichia coli. ''Molecular Microbiology, 78(2)'', 320-330. </ref>. The drug molecule targeted for excretion is captured by the AcrB subunit (most likely from the periplasm or from the periplasm-intermembrane interface) and is then passed on to the TolC complex for final export. Of course, one could argue that the most important member of the AcrAB-TolC resistance complex is the member that is responsible for the initial attraction of the target compound, | + | Certain bacteria (''Escherichia coli'', for example) contain a proton motive force-dependent multidrug <scene name='Ciprofloxacin/Cv/1'>efflux pump</scene>, which, as the name suggests, grants the bacteria resistance to certain drugs <ref>Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. ''Journal of Bacteriology, 188(20)'', 7290-7296. </ref>. In ''Escherichia coli'', the efflux system that confers particular drug resistance is a tripartite transmembrane resistance structure known as "AcrAB-TolC" <ref>Husain, F., Nikaido, H. (2010). Substrate path in the AcrB multidrug efflux pump of Escherichia coli. ''Molecular Microbiology, 78(2)'', 320-330. </ref>. The drug molecule targeted for excretion is captured by the AcrB subunit (most likely from the periplasm or from the periplasm-intermembrane interface) and is then passed on to the TolC complex for final export. Of course, one could argue that the most important member of the AcrAB-TolC resistance complex is the member that is responsible for the initial attraction of the target compound, the AcrB subunit. Ciprofloxacin may be <scene name='Sandbox_100/Orientation_of_cipro_on_acrb/1'>captured by the AcrB subunit</scene> for removal from the bacterial cell (in this scene, AcrB is in the proposed transmembrane orientation assuming lower cytosolic face and upper exoplasmic face). It has been shown that <scene name='Sandbox_100/Phe_residues/1'> Phe 386 and Phe 388</scene> contribute to the effectiveness of the initial affinity of AcrB for all targets <ref>Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. ''Journal of Bacteriology, 188(20)'', 7290-7296. </ref> (in this scene, both Phe residues are magenta). It has also been shown that, after ligand binding, a proton may bind to acidic residue in the transmembrane domain, which contains an as yet putative network of electrostatically interacting residues, the perturbation of which interacting residues leads to a series of conformational changes that result in drug expulsion. Residues involved in this chain of events include <scene name='Sandbox_100/Asp_407_408_efflux/1'>Asp 407, Asp 408</scene>, <scene name='Sandbox_100/Lys_940_efflux/1'>Lys 940</scene> and <scene name='Sandbox_100/Thr_178_efflux/1'>Thr 978</scene> (red, purple, green, respectively). The precise mechanism of the action of the AcrB efflux subunit (and of the tripartite AcrAB-TolC in general) is still under scrutiny. |
{{Clear}} | {{Clear}} | ||
== Conclusion == | == Conclusion == | ||
Revision as of 15:47, 26 June 2013
| |||||||||||
References
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ 2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.
- ↑ 2001. Information on Cipro (Ciprofloxacin Hydrochloride) for Inhalation Anthrax for Consumers: Questions and Answers. Fda.gov. http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130711.htm. Last updated, 2009.
- ↑ 2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.
- ↑ Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.
- ↑ Ciprofloxacin - Activity, Business Aspects/Bayer Pharmaceutical. Encyclopedia.jrank.org. http://encyclopedia.jrank.org/articles/pages/1398940/Ciprofloxacin.html
- ↑ Siegmund, K., et al. (2005). Molecular details of quinolone-DNA interactions: solution structure of an unusually stable DNA duplex with covalently linked nalidixic acid residues and non-covalent complexes derived from it. Nucleic Acids [Research], 33(15), 4838-4848.
- ↑ Peterson, L. (2001). Quinolone-Molecular Structure-Activity Relationships: What We Have Learned About Improving Antimicrobial Activity. Clinical Infectious Diseases, 33(3), S180-S186.
- ↑ Image from: http://cid.oxfordjournals.org/content/33/Supplement_3/S180.full.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Molecular weight from Chemexper.com.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://textbookofbacteriology.net/themicrobialworld/cipro.gif&imgrefurl=http://textbookofbacteriology.net/themicrobialworld/control.html&usg=__wtzKLHB3NssfnODEB224br5-Bcw=&h=200&w=250&sz=2&hl=en&start=0&zoom=1&tbnid=o7VT7s6FFIUrWM:&tbnh=160&tbnw=199&ei=Hk10TaypBcL58AbyvIjKDw&prev=/images%3Fq%3Dciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=527&vpy=300&dur=1709&hovh=160&hovw=200&tx=155&ty=82&oei=EU10TcvOCMbdtge5msiLDw&page=1&ndsp=16&ved=1t:429,r:7,s:0.
- ↑ Molecular weight from: CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://images.rxlist.com/images/rxlist/ciloxan_s.gif&imgrefurl=http://www.rxlist.com/ciloxan_ophthalmic_ointment-drug.htm&usg=__UqTKseSe8hD85c5RLGIz2_dbAg0=&h=142&w=232&sz=2&hl=en&start=16&zoom=1&tbnid=70Q2WG5hppsQ5M:&tbnh=100&tbnw=164&ei=T010TenMFYH_8Aa6gvDKDw&prev=/images%3Fq%3Dciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:10%2C624&um=1&itbs=1&iact=hc&vpx=1064&vpy=399&dur=309&hovh=106&hovw=174&tx=98&ty=76&oei=EU10TcvOCMbdtge5msiLDw&page=2&ndsp=18&ved=1t:429,r:17,s:16&biw=1280&bih=647.
- ↑ Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://www.chemdrug.com/databases/SYNTHESIS/SYN/09/09000601a.gif&imgrefurl=http://www.chemdrug.com/databases/8_0_dvpytumicutbciwa.html&usg=__TxiDuzCve6C_crxmcPYTpfW5d4s=&h=555&w=678&sz=6&hl=en&start=0&zoom=1&tbnid=xhquLksJBbMnjM:&tbnh=165&tbnw=201&ei=0Y93TdbGI-yI0QGspa25Bw&prev=/images%3Fq%3Dsynthesis%2Bof%2Bciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=346&vpy=105&dur=63&hovh=203&hovw=248&tx=170&ty=128&oei=0Y93TdbGI-yI0QGspa25Bw&page=1&ndsp=16&ved=1t:429,r:1,s:0
- ↑ Ciprofloxacin Oral - Monograph - Ciprofloxacin Hydrochloride. 2009. Medscape.com. http://www.medscape.com/druginfo/monograph cid=med&drugid=7748&drugname=Ciprofloxacin+Oral&monotype=monograph&secid=8.
- ↑ Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. Journal of Bacteriology, 188(20), 7290-7296.
- ↑ Husain, F., Nikaido, H. (2010). Substrate path in the AcrB multidrug efflux pump of Escherichia coli. Molecular Microbiology, 78(2), 320-330.
- ↑ Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. Journal of Bacteriology, 188(20), 7290-7296.
Proteopedia Page Contributors and Editors (what is this?)
John Ripollone, John E. Ripollone, Alexander Berchansky, David Canner