Sandbox Reserved 775
From Proteopedia
(→Nisin) |
|||
| Line 4: | Line 4: | ||
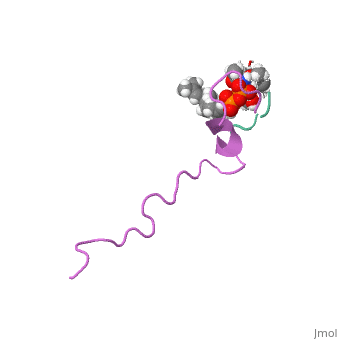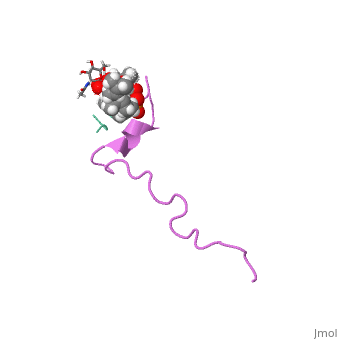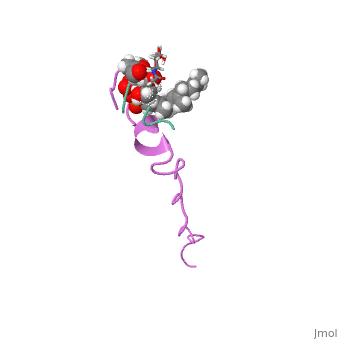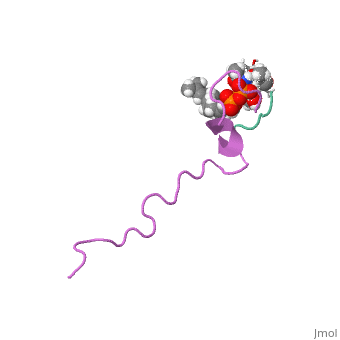
<Structure load='1wco' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | <Structure load='1wco' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
| - | == | + | == Introduction == |
Nisin is a broad spectrum bacteriocin produced by the bacterium Lactococcus lactis. It is currently used in the food industry as a natural preservative (Burrowes and others 2004). It is often used as an antimicrobial agent in processed cheese to prevent the growth of various Clostridium bacteria that were present in the initial ingredients that survived the heat treatment of the cheese. In addition to processed cheese, Nisin is used in many other food products such as salad dressing, alcoholic beverages, canned vegetables, and meat (Delves-Broughton 2005). | Nisin is a broad spectrum bacteriocin produced by the bacterium Lactococcus lactis. It is currently used in the food industry as a natural preservative (Burrowes and others 2004). It is often used as an antimicrobial agent in processed cheese to prevent the growth of various Clostridium bacteria that were present in the initial ingredients that survived the heat treatment of the cheese. In addition to processed cheese, Nisin is used in many other food products such as salad dressing, alcoholic beverages, canned vegetables, and meat (Delves-Broughton 2005). | ||
Revision as of 15:40, 15 November 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
|
Introduction
Nisin is a broad spectrum bacteriocin produced by the bacterium Lactococcus lactis. It is currently used in the food industry as a natural preservative (Burrowes and others 2004). It is often used as an antimicrobial agent in processed cheese to prevent the growth of various Clostridium bacteria that were present in the initial ingredients that survived the heat treatment of the cheese. In addition to processed cheese, Nisin is used in many other food products such as salad dressing, alcoholic beverages, canned vegetables, and meat (Delves-Broughton 2005).
Background
Bacteriocins are antimicrobial peptides produced by bacteria to inhibit other closely related strains. These chemicals are distinguished from antibiotics in various ways. Bacteriocins are synthesized in the ribosome with a narrow activity spectrum while antibiotics are secondary metabolites and their spectrums vary. Bacteriocins do not harm the host cell and, unlike antibiotics, have no known toxicity to humans. Nisin is the only FDA approved bacteriocin currently used in food (Burrowes and others 2004). Unusually, it has a broad spectrum of activity, as it inhibits many gram positive bacteria. While nisin is effective against many gram positive bacteria, it is ineffective at inhibiting gram negative strains (Delves-Broughton 2005).
Mode of Action
This peptide inhibits bacteria by creating pores in the cytoplasmic membrane, which disrupts the proton motive force. There are three proposed models of inhibition: barrel-stave, torroidal, and carpet (Figure 1) (Bechinger and Lohner 2006). For the barrel-stave and torroidal models, it is thought that the positively charged peptide is electrostatically attracted to the negatively charged cytoplasmic membrane. Once the peptide comes into contact with the membrane, the peptide folds itself into an alpha helical structure, and when a high enough concentration of peptides adsorbs onto the membrane surface, a pore forms in the following manner. In the barrel-stave model, the peptides form a very ordered barrel like structure through the membrane creating a pore that intercellular fluids can leak out of (Bechinger and Lohner 2006). In the torroidal pore model, pore formation occurs either through the AMP imbedding itself into the membrane or through positive curvature strain (Bechinger and Lohner 2006). The pores formed through either of these models can cause leaking of intercellular fluid and the loss of membrane potential. In the carpet model, AMP’s aggregate onto the bacterial membrane much like the barrel stave and the torroidal model; but instead of imbedding themselves into the membrane; the peptides form a carpet like structure on the outer surface of the membrane by electrostatically binding to the phospholipid head group and directly dissolving the membrane (Oren and Shai 1998). The structure causes the membrane to break into pieces through micellization (Bechinger and Lohner 2006). Non-membrane targeting AMP’s pass through the bacterial membrane without damaging it and have been observed inhibiting protein synthesis, nucleic acid synthesis, enzymatic activity, and cell wall synthesis (Zhang and others 2001).