Sandbox Reserved 771
From Proteopedia
| Line 53: | Line 53: | ||
| - | == | + | ==Glutathione Deficiency Syndrome== |
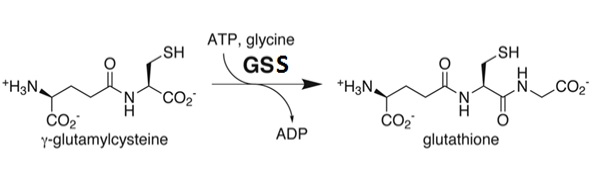
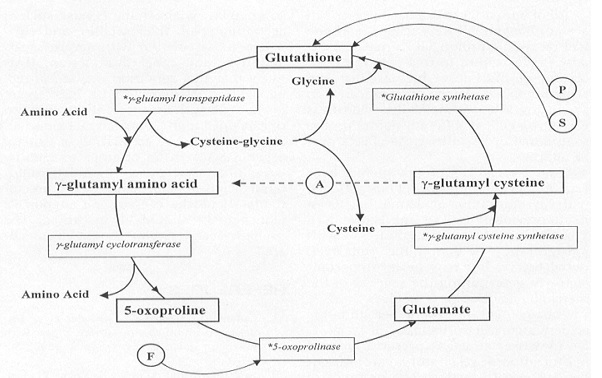
| - | Though reduced levels of GSH have been observed in patients with Alzheimers and Parkinsons, inborn errors in the endogenous GSS enzyme resulting in significantly low levels of GSH is believed to be the cause of a metabolic deficiency termed 5-oxoprolinuria. | + | Though reduced levels of GSH have been observed in patients with Alzheimers and Parkinsons, inborn errors in the endogenous GSS enzyme resulting in significantly low levels of GSH is believed to be the cause of a very rare metabolic deficiency in which there is a large build up of 5-oxoproline in the urine - termed 5-oxoprolinuria. It is so rare that, as of 2006, it had been diagnosed in less than 100 people worldwide. |
| + | |||
| + | GSH deficiency has been sub-divided into three forms: Mild, Moderate, and Severe. Mild deficiencies usually result in what is known as hemolytic anemia, and is a result of the degradation of red blood cells. Rarely, mild GSH deficiencies can result in 5-oxoprolinuria. More moderate GSS deficiencies will result in a higher likelihood of developing 5-oxoprolinuria, hemolytic anemia, and metabolic acidosis - a condition in which the blood pH is lower than the homeostatic pH of 7 - shortly after birth. Finally, individuals with severe GSH deficiencies experience severe neurological symptoms in addition to those associated with moderate GSH deficiencies. Slowed physical movements, reactions, and speech, as well as intellectual retardation and a loss of coordination are those frequently associated with severe GSH deficiency. | ||
| + | |||
| + | Studies suggest that the rarity of this disorder can be attributed to the fact it is autosomal recessive and thus both copies of the cell's chromosome must contain the genetic coding for the disorder. Each of the parents must carry a single copy of the mutated <i>gss gene, thus displaying no physical symptoms, and both must pass their mutated copy on to the child. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 02:07, 5 December 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
| |||||||||||
References
1. http://www.ncbi.nlm.nih.gov/protein/NP_000169.1
2. Breton CV, Salam MT, Vora H, Gauderman WJ, Gilliland FD. 2011. Genetic variation in the glutathione synthesis pathway, air pollution, and children's lung function growth. Amer Jour Respir Crit Care Med, 183(2): 243-248. doi: 10.1164/rccm.201006-0849OC
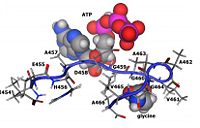
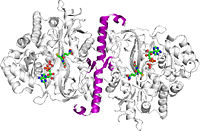
3. Brown TR, Drummond ML, Barelier S, Crutchfield AS, Dinescu A, Slavens KD, Cundari TR, Anderson ME. 2011. Asparate 458 of human glutathione synthetase is importatnt for cooperativity and active site structure. Biochem & Biophys Resear Comm, 411(3): 536-542. doi: 10.1016/j.bbrc.2011.06.166
4. Dinescu A, Brown TR, Barelier S, Cundari TR, Anderson ME. 2010. The role of the glycine triad in human glutathione synthesis. Biochem Biophys Res Commun, 400(4):511-516. doi: 10.1016/j.bbrc.2010.08.081
5. Slavens KD, Brown TR, Barakat KA, Cundari TR, Anderson ME. 2011. Valine 44 and valine 45 of human glutathione synthetase are key for subunit stability and negative cooperativity. Biochem & Biophys Resear Comm, 410(3): 597-601. doi: 10.1016/j.bbrc.2011.06.034
6. Uchida M, Sugaya M, Janamary T, Hisatomi H. 2010. Alternative RNA splicing in expression of the glutathione synthetase gene in human cells. Mol Biol Rep, 37(4): 2105-2109. doi: 10.1007/s11033-009-9675-3