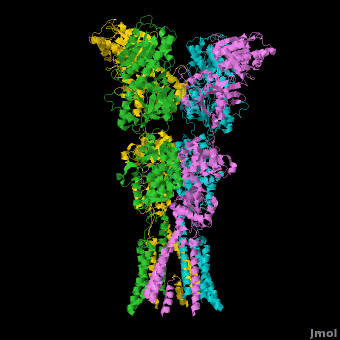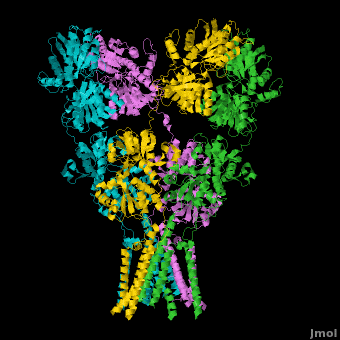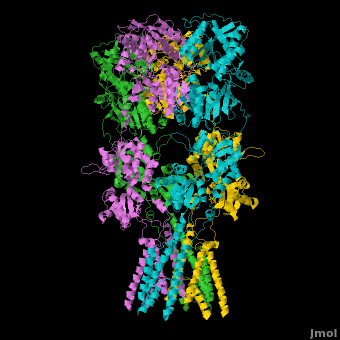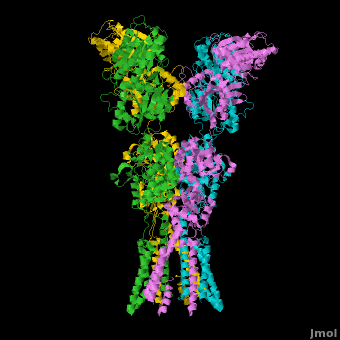Ionotropic Glutamate Receptors
From Proteopedia
| Line 20: | Line 20: | ||
====Pharmaceutical Relevance==== | ====Pharmaceutical Relevance==== | ||
As mentioned previously, extensive investigation into the [[Pharmaceutical drugs|pharmaceutical potential]] of IGluRs as a target for treating various ailments including [[Autism Spectrum Disorders]] symptoms is ongoing. In addition to agents which reduce neural excitation such as benzodiazapines, small molecules that potentiate AMPA receptor currents have been proven to reduce cognitive deficits caused by neurodegenerative diseases such as [[Alzheimer's Disease]].<ref name="Purcel"/> Modulators such as aniracetam and CX614 <scene name='Ionotropic_Glutamate_Receptors/Locked_into_place/2'>bind on the backside</scene> ([[2al4]]) of the ligand-binding core through interactions with a “proline ceiling” and a “serine floor”, stabilizing the closed-clamshell conformation. Although these compounds would likely be ineffective in the case of Autism patients because they slow the deactivation of the IGluR channels, this class of compounds has exciting therapeutic potential.<ref name="Jin"/> | As mentioned previously, extensive investigation into the [[Pharmaceutical drugs|pharmaceutical potential]] of IGluRs as a target for treating various ailments including [[Autism Spectrum Disorders]] symptoms is ongoing. In addition to agents which reduce neural excitation such as benzodiazapines, small molecules that potentiate AMPA receptor currents have been proven to reduce cognitive deficits caused by neurodegenerative diseases such as [[Alzheimer's Disease]].<ref name="Purcel"/> Modulators such as aniracetam and CX614 <scene name='Ionotropic_Glutamate_Receptors/Locked_into_place/2'>bind on the backside</scene> ([[2al4]]) of the ligand-binding core through interactions with a “proline ceiling” and a “serine floor”, stabilizing the closed-clamshell conformation. Although these compounds would likely be ineffective in the case of Autism patients because they slow the deactivation of the IGluR channels, this class of compounds has exciting therapeutic potential.<ref name="Jin"/> | ||
| - | + | ||
__NOEDITSECTION__ | __NOEDITSECTION__ | ||
</StructureSection> | </StructureSection> | ||
Revision as of 09:51, 18 August 2014
| |||||||||||
Page Development
This article was developed based on lectures given in Chemistry 543 by Prof. Clarence E. Schutt at Princeton University.
3D structures of glutamate receptor
Updated on 18-August-2014
Topic Page on Glutamate Receptor GluA2 structure
There is a topic page describing in detail the GluA2 structure described in 3kg2. The page is meant to complement the original publication of the structure by Sobolevsky et al.[2][8] with matching colors, etc..
Ionotropic glutamate receptor 0
2pyy - IGluR2 ligand-binding domain + Glu – Nostoc punctiforme
Ionotropic glutamate receptor 1
3saj - rIGluR1 N-terminal domain – rat
Ionotropic glutamate receptor 2
3n6v, 3o2j - rIGluR2 N-terminal domain (mutant) – rat
3hsy, 3h5v, 3h5w - rIGluR2 N-terminal domain
2wjw, 2wjx – hIGluR2 N-terminal domain - human
3rn8, 3rnn – hIGluR2 ligand-binding domain + potentiator
3bki - rIGluR2 ligand-binding domain + inhibitor
1fto, 4h8j - rIGluR2 ligand-binding domain
3t93 - rIGluR2 ligand-binding domain (mutant)
GluR2 positive allosteric modulator complex
2xhd - hIGluR2 ligand-binding domain + positive allosteric modulator + Glu
2al4, 1p1o - rIGluR2 ligand-binding domain (mutant) + positive allosteric modulator
2xx8, 2xx7, 2xx9, 2xxh, 2xxi, 3lsf, 3h6t, 3h6u, 3h6v, 3h6w, 3bbr, 3tkd, 4fat, 4iy5, 4iy6, 4lz5, 4lz7, 4lz8, 4n07 - rIGluR2 ligand-binding domain (mutant) + positive allosteric modulator + Glu
3pmv, 3pmw, 3pmx, 3o28, 3o29, 3o2a, 3o6g, 3o6h, 3o6i, 3m3l, 3lsl, 3tdj - rIGluR2 ligand-binding domain + positive allosteric modulator + Glu
1mm6, 1mm7 - rIGluR2 ligand-binding domain + positive allosteric modulator
3ijo, 3ijx, 3ik6, 3il1, 3ilt, 3ilu - rIGluR2 + positive allosteric modulator + Glu
GluR2 antagonist complex
3r7x - hIGluR2 + antagonist
3kgc, 3kg2, 2cmo - rIGluR2 ligand-binding domain + antagonist + Glu
A topic page describing in detail the GluA2 structure described in 3kg2
3h03, 3h06, 3b7d, 1n0t, 1ftl, 3tza, 3ua8, 4isu - rIGluR2 ligand-binding domain + antagonist
1lb9, 4l17 - rIGluR2 ligand-binding domain (mutant) + antagonist
GluR2 agonist complex
3rtf, 3rtw, 3pd8, 3pd9, 3bft, 3bfu, 1wvj, 1syh, 1syi, 1ms7, 1mqd, 1nnp, 1nnk, 1m5b, 1m5c, 1m5d, 1m5e, 1m5f, 1ftm, 4g8m, 4igt - rIGluR2 ligand-binding domain + agonist
3b6t, 2al5, 2anj, 1p1q, 1p1u, 1p1w, 1lb8 - rIGluR2 ligand-binding domain (mutant) + agonist
1lbc - rIGluR2 ligand-binding domain (mutant) + agonist + Glu
2p2a - rIGluR2 ligand-binding domain + agonist + Glu
GluR2 partial agonist complex
1y1m, 2aix, 1y1z, 1y20, 1mqg, 1mqh, 1mqi, 1mqj, 1mxu, 1mxv, 1mxw, 1mxx, 1mxy, 1mxz, 1my0, 1my1, 1my2, 1my3, 1my4, 1fw0, 1ftk, 1gr2 - rIGluR2 ligand-binding domain + partial agonist
1xhy, 1p1n, 1lbb, 3t96, 3t9h, 3t9u, 3t9v - rIGluR2 ligand-binding domain (mutant) + partial agonist
GluR2 ligand complex
3dp6, 2uxa, 2i3v, 2i3w, 1ftj - rIGluR2 ligand-binding domain + Glu
3b6q, 3b6w, 2gfe, 3t9x - rIGluR2 ligand-binding domain (mutant) + Glu
4gxs - rIGluR2 ligand-binding domain + kainate derivative
Ionotropic glutamate receptor 3
3o21, 3p3w – rIGluR3 N-terminal domain
3m3k – rIGluR3 ligand-binding domain
3rt6, 3rt8, 3dp4 – rIGluR3 ligand-binding domain + agonist
3m3f – rIGluR3 ligand-binding domain + allosteric modulator
3dln - rIGluR3 ligand-binding domain + Glu
4f1y - rIGluR3 ligand-binding domain + CNQX
4f22, 4f39, 4f3g - rIGluR3 ligand-binding domain + kainate
4f31 - rIGluR3 ligand-binding domain (mutant) + kainate
4f29 - rIGluR3 ligand-binding domain + quisqualate
4f2o, 4f2q - rIGluR3 ligand-binding domain (mutant) + quisqualate
4f3b - rIGluR3 ligand-binding domain (mutant) + Glu
Ionotropic glutamate receptor 4
3epe, 3fas - rIGluR4 ligand-binding domain + Glu
3kei – rIGluR4 ligand-binding domain (mutant) + Glu
3kfm - rIGluR4 ligand-binding domain (mutant) + partial agonist
3en3 - rIGluR4 ligand-binding domain + partial agonist
3fat – rIGluR4 ligand-binding domain + agonist
4gpa – rIGluR4 N terminal domain
Ionotropic glutamate receptor 5
3fuz, 2zns – hIGluR5 ligand-binding domain + Glu
1txf - rIGluR5 ligand-binding domain + Glu
2ojt - rIGluR5 + anion
3fv1, 3fv2, 3fvg, 3fvk, 3fvn, 3fvo, 2znt, 2znu - hIGluR5 ligand-binding domain + agonist
3c31, 3c32, 3c33, 3c34, 3c35, 3c36 - rIGluR5 ligand-binding domain + ion
2wky, 3gba, 3gbb, 2pbw, 2f34, 2f35, 2f36 – rIGluR5 ligand-binding domain + agonist
2qs1, 2qs2, 2qs3, 2qs4 - rIGluR5 ligand-binding domain (mutant) + agonist
1vso - rIGluR5 ligand-binding domain + antagonist
Ionotropic glutamate receptor 6
3h6g, 3h6h – rIGluR6 N-terminal domain
3g3f, 1sd3, 1s50, 1s7y – rIGluR6 ligand-binding domain + Glu
3g3g, 3g3h, 3g3i, 3g3j, 3g3k, 2i0b, 2i0c - rIGluR6 ligand-binding domain (mutant) + Glu
1s9t – rIGluR6 ligand-binding domain + positive allosteric modulator
1tt1 – rIGluR6 ligand-binding domain + partial agonist
Metabotropic glutamate receptor
Metabotropic glutamate receptor
Ionotropic kainate receptor 1
1ycj – rGluK1 ligand-binding domain + Glu
3s2v, 4dld – rGluK1 ligand-binding domain + antagonist
4e0x – rGluK1 ligand-binding domain + kainate
Ionotropic kainate receptor 2
2xxr – rGluK2 ligand-binding domain + Glu
4h8i - rGluK2 ligand-binding domain + Glu derivative
2xxu, 2xxx, 2xxw, 4bdl, 4bdn, 4bdq – rGluK2 ligand-binding domain (mutant) + Glu
4bdm, 4bdo, 4bdr – rGluK2 ligand-binding domain (mutant) + kainate
2xxt – rGluK2 ligand-binding domain + partial agonist
2xxv, 2xxy – rGluK2 ligand-binding domain (mutant) + partial agonist
3qlt - rGluK2 residues 32-420 (mutant)
1yae - rGluK2 ligand-binding domain + agonist
3qxm - hGluK2 ligand-binding domain + toxin
Ionotropic kainate receptor 3
3olz – rGluK3 N-terminal domain
3s9e, 3u93, 3u94, 4mh5 – rGluK3 ligand-binding domain + Glu
3u92, 4e0w - rGluK3 ligand-binding domain + kainate
4g8n, 4igr - rGluK3 ligand-binding domain + agonist
Ionotropic kainate receptor 5
3om0, 3om1 – rGluK5 N-terminal domain
3qlu - rGluK5 + rGluK2 (mutant)
3qlv - rGluK5 + rGluK2
NMDA receptor
3jpy, 3jpw – rNMDA subunit ε2 N-terminal domain (mutant)
2a5t - rNMDA subunits NR1 and NR2A ligand-binding domains
2a5s - rNMDA subunits NR1 and NR2A ligand-binding domains + Glu
3qek – XlNMDA subunit GLUN1 N terminal (mutant) – Xenopus laevis
3qel, 3qem – XlNMDA subunit GLUN1 N terminal (mutant) + rNMDA subunit ε2 N-terminal domain (mutant) + ifenprodil
4kcc – rNMDA 1 ligand-binding domain
4jwy – rNMDA 2D ligand-binding domain + agonist
4kcd – rNMDA 3A ligand-binding domain
4kfq – rNMDA 1 ligand-binding domain + antagonist
4nf4, 4nf5, 4nf6, 4nf8 – rNMDA 1 ligand-binding domain + rNMDA 2A ligand-binding domain (mutant)
See Also
- Glutamate Receptor Symmetry Analysis
- Glutamate receptor (GluA2) structure in detail
- Metabotropic glutamate receptor
- Membrane Channels & Pumps
- Alzheimer's Disease
References
- ↑ 1.0 1.1 1.2 Jin R, Clark S, Weeks AM, Dudman JT, Gouaux E, Partin KM. Mechanism of positive allosteric modulators acting on AMPA receptors. J Neurosci. 2005 Sep 28;25(39):9027-36. PMID:16192394 doi:25/39/9027
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009 Dec 10;462(7274):745-56. Epub . PMID:19946266 doi:10.1038/nature08624
- ↑ 3.0 3.1 3.2 3.3 Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001 Nov 13;57(9):1618-28. PMID:11706102
- ↑ Welsh JP, Ahn ES, Placantonakis DG. Is autism due to brain desynchronization? Int J Dev Neurosci. 2005 Apr-May;23(2-3):253-63. PMID:15749250 doi:10.1016/j.ijdevneu.2004.09.002
- ↑ Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 1997 Aug 21;388(6644):769-73. PMID:9285588 doi:10.1038/42009
- ↑ Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003 Oct;2(5):255-67. PMID:14606691
- ↑ Jin R, Singh SK, Gu S, Furukawa H, Sobolevsky AI, Zhou J, Jin Y, Gouaux E. Crystal structure and association behaviour of the GluR2 amino-terminal domain. EMBO J. 2009 Jun 17;28(12):1812-23. Epub 2009 May 21. PMID:19461580 doi:10.1038/emboj.2009.140
- ↑ Wollmuth LP, Traynelis SF. Neuroscience: Excitatory view of a receptor. Nature. 2009 Dec 10;462(7274):729-31. PMID:20010675 doi:10.1038/462729a
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Wayne Decatur, Alexander Berchansky, Joel L. Sussman