Lauren Ferris/Sandbox 2
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
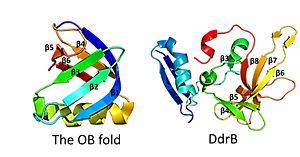
This is followed by <scene name='57/578563/4exw_monomer_6b/1'>6 beta strands</scene>, which contain a solvent exposed face and another face that against the N-terminal motif. The beta sheets are anti-parallel and do not form an OB fold as determined by multiple servers iCOPS, DALI, 3D-BLAST, and MATRAS.<ref>PMID: 20129942</ref> At the time of this finding (2010), the lack of an OB fold was surprising, since all ssDNA binding proteins were thought to bind to DNA through an OB fold. The OB fold is two three-stranded anti-parallel β sheets that form a five stranded β barrel. The OB folds adopt greek key motifs. The differences between the DdrB beta strands and those in the OB fold include the topology of the β strands. DdrB strands form an up and down topology and not a Greek key. Furthermore, monomeric DdrB β strands do not form a beta barrel. Additionally, DdrB has different connectivity, no conserved glycine, and no β bulge.<ref>PMID: 20129942</ref> [[Image:OB fold.jpg|300px|left|thumb|This figure highlights the differences between the classic OB fold found in single-stranded binding proteins and the novel structural features of DdrB. The OB fold is observed in the protein verotoxin-1, PDB code [[2XSC]] . DdrB is modeled from the PDB structure [[4HQB]]. This figure was generated using Pymol.]] | This is followed by <scene name='57/578563/4exw_monomer_6b/1'>6 beta strands</scene>, which contain a solvent exposed face and another face that against the N-terminal motif. The beta sheets are anti-parallel and do not form an OB fold as determined by multiple servers iCOPS, DALI, 3D-BLAST, and MATRAS.<ref>PMID: 20129942</ref> At the time of this finding (2010), the lack of an OB fold was surprising, since all ssDNA binding proteins were thought to bind to DNA through an OB fold. The OB fold is two three-stranded anti-parallel β sheets that form a five stranded β barrel. The OB folds adopt greek key motifs. The differences between the DdrB beta strands and those in the OB fold include the topology of the β strands. DdrB strands form an up and down topology and not a Greek key. Furthermore, monomeric DdrB β strands do not form a beta barrel. Additionally, DdrB has different connectivity, no conserved glycine, and no β bulge.<ref>PMID: 20129942</ref> [[Image:OB fold.jpg|300px|left|thumb|This figure highlights the differences between the classic OB fold found in single-stranded binding proteins and the novel structural features of DdrB. The OB fold is observed in the protein verotoxin-1, PDB code [[2XSC]] . DdrB is modeled from the PDB structure [[4HQB]]. This figure was generated using Pymol.]] | ||
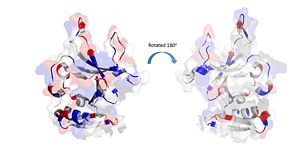
| - | Positively Charged amino acids reside in the solvent exposed beta strands, which may potentially enable the binding of ssDNA. [[Image:electrostatics monomer.jpg|300px|left|thumb|]] | + | Positively Charged amino acids reside in the solvent exposed beta strands, which may potentially enable the binding of ssDNA. [[Image:electrostatics monomer.jpg|300px|left|thumb|The electrostatic charges in the DdrB monomer. Blue represents positive charges, while red represents negative charges. (PDB: [[4HQB]]). This figure was generated using Pymol.]] |
<scene name='57/578563/4exw_monomer_loops/1'>Two loops</scene> that link beta sheet 6 to sheet 7 and beta sheet 7 to sheet 8 contain flexible regions with poor order as determined by limited to no density in the crystal structure. This finding suggests that these loops are intrinsically disordered.<ref>PMID: 20129942</ref> | <scene name='57/578563/4exw_monomer_loops/1'>Two loops</scene> that link beta sheet 6 to sheet 7 and beta sheet 7 to sheet 8 contain flexible regions with poor order as determined by limited to no density in the crystal structure. This finding suggests that these loops are intrinsically disordered.<ref>PMID: 20129942</ref> | ||
| Line 38: | Line 38: | ||
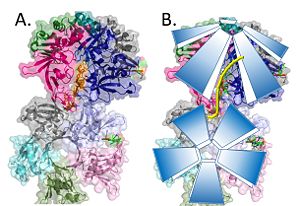
The monomeric units of DdrB collectively form a <scene name='57/578563/4exw_pentamer/1'>pentameric ring </scene>with a 10 A pore in the center of this structure.<ref>PMID: 20129942</ref> Other DNA binding proteins can thread DNA through a central pore, however this seems highly unlikely in the case of DdrB. The pore size appears too small (would need to be 14-40A) and has a net negative charge making it highly unfavorable for DNA interactions.<ref>PMID: 20129942</ref> The beta-beta-alpha motif at the N-terminus of the monomer facilitates the formation of this <scene name='57/578563/Start_molecule_and_bbar/1'> pore </scene> as the beta sheets of the N-terminal beta-beta-alpha motif form a 10 stranded <scene name='57/578563/B_barrel/1'>anti-parallel B-barrel</scene>. This structure is stabilized by interactions with the alpha helices. <ref>PMID: 20129942</ref> | The monomeric units of DdrB collectively form a <scene name='57/578563/4exw_pentamer/1'>pentameric ring </scene>with a 10 A pore in the center of this structure.<ref>PMID: 20129942</ref> Other DNA binding proteins can thread DNA through a central pore, however this seems highly unlikely in the case of DdrB. The pore size appears too small (would need to be 14-40A) and has a net negative charge making it highly unfavorable for DNA interactions.<ref>PMID: 20129942</ref> The beta-beta-alpha motif at the N-terminus of the monomer facilitates the formation of this <scene name='57/578563/Start_molecule_and_bbar/1'> pore </scene> as the beta sheets of the N-terminal beta-beta-alpha motif form a 10 stranded <scene name='57/578563/B_barrel/1'>anti-parallel B-barrel</scene>. This structure is stabilized by interactions with the alpha helices. <ref>PMID: 20129942</ref> | ||
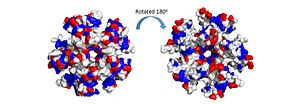
| - | The pentamer also contains a positive residue track on one side of the pentamer. These residues facilitate ssDNA binding and DdrB functionality. <ref>PMID: 20129942</ref> [[Image:electrostatics pentamer.jpg|300px|left|thumb|]] | + | The pentamer also contains a positive residue track on one side of the pentamer. These residues facilitate ssDNA binding and DdrB functionality. <ref>PMID: 20129942</ref> [[Image:electrostatics pentamer.jpg|300px|left|thumb|The electrostatic charges in the DdrB pentamer. Blue represents positive charges, while red represents negative charges. A positive “track” around the top of the pentamer may enable ssDNA binding to one side of the pentamer (left image). (PDB: [[4HQB]]). This figure was generated using Pymol.]] |
| Line 44: | Line 44: | ||
Recently, a structure for Deinococcus radiodurans in complex with ssDNA has been solved.<ref>PMID: 23975200 </ref> The structure revealed that ssDNA bind in two clefts between three subunits. One 4 base pair strand of DT bound in the cleft between E and A, while the other 4 base pair strand of DT bound between A and B. Interestingly, when symmetry was applied to the crystal the dT strands appeared continuous. <ref>PMID: 23975200 </ref> | Recently, a structure for Deinococcus radiodurans in complex with ssDNA has been solved.<ref>PMID: 23975200 </ref> The structure revealed that ssDNA bind in two clefts between three subunits. One 4 base pair strand of DT bound in the cleft between E and A, while the other 4 base pair strand of DT bound between A and B. Interestingly, when symmetry was applied to the crystal the dT strands appeared continuous. <ref>PMID: 23975200 </ref> | ||
| - | [[Image:Pentamer-pentamer.jpg|300px|left|thumb|]] | + | [[Image:Pentamer-pentamer.jpg|300px|left|thumb|Model of the pentamer-pentamer structure. (PDB: [[4HQB]]) This figure was generated using Pymol.]] |
| Line 52: | Line 52: | ||
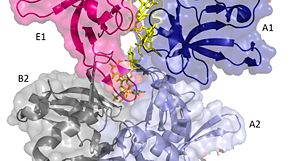
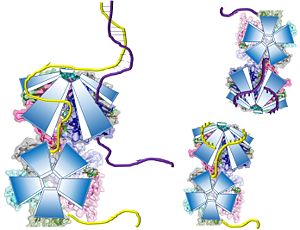
The crystal structure also determined that the pentameric ring binds to another pentameric ring. Given the continuous chain formed by the DT strands it appears that the chain passes through the channel formed by subunits A and E in one pentamer. (which will now be referred to as A1 and E1). To pass through the channel in the second pentamer between A and B (now A2 and B2). In this direction the DNA strand runs in the 5’ to 3’ direction.<ref>PMID: 23975200 </ref> | The crystal structure also determined that the pentameric ring binds to another pentameric ring. Given the continuous chain formed by the DT strands it appears that the chain passes through the channel formed by subunits A and E in one pentamer. (which will now be referred to as A1 and E1). To pass through the channel in the second pentamer between A and B (now A2 and B2). In this direction the DNA strand runs in the 5’ to 3’ direction.<ref>PMID: 23975200 </ref> | ||
| - | [[Image:DNA channel.jpg|300px|left|thumb|]] | + | [[Image:DNA channel.jpg|300px|left|thumb|Two pentamers associate forming a channel for the ssDNA to thread through. (PDB: [[4HQB]]) This figure was generated using Pymol.]] |
| Line 73: | Line 73: | ||
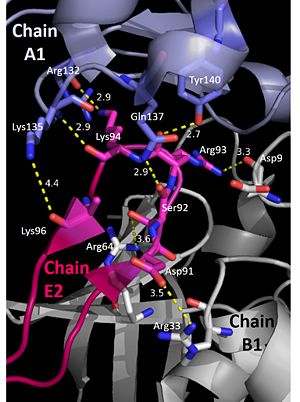
The interactions between DdrB and the DNA strand include: | The interactions between DdrB and the DNA strand include: | ||
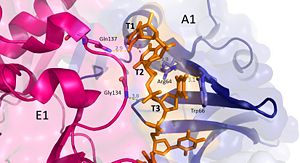
| - | [[Image:t1-3.jpg|300px|left|thumb|]] | + | [[Image:t1-3.jpg|300px|left|thumb|Interactions between DdrB and T1-T3. (PDB: [[4HQB]]) This figure was generated using Pymol.]] |
'''Nucleotides 1, 2, 3 (T1, T2, and T3 respectively):''' Interact with Chains A1 and E1 | '''Nucleotides 1, 2, 3 (T1, T2, and T3 respectively):''' Interact with Chains A1 and E1 | ||
| Line 79: | Line 79: | ||
T3 hydrogen bonds to R64 of chain A1, forms a pi-pi interaction with W66 from chain A. T3 also forms hydrogen bonds with the 5’ phosphate and amino group of G134 in chain E1. <ref>PMID: 23975200 </ref> | T3 hydrogen bonds to R64 of chain A1, forms a pi-pi interaction with W66 from chain A. T3 also forms hydrogen bonds with the 5’ phosphate and amino group of G134 in chain E1. <ref>PMID: 23975200 </ref> | ||
| + | |||
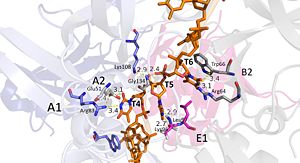
| + | [[Image:DNA T4-6.jpg|300px|left|thumb|Interactions between DdrB and T4-T6. (PDB: [[4HQB]]) This figure was generated using Pymol.]] | ||
'''Nucleotides 4, 5, 6 (T4, T5, and T6 respectively):''' Bridge the Pentamers | '''Nucleotides 4, 5, 6 (T4, T5, and T6 respectively):''' Bridge the Pentamers | ||
T4 stabilized by electrostatic interactions with R83 from chain A and E51 from chain A. T5 forms hydrogen bonds with K96 of Chain E and forms van der Waal contacts with K135 chain A. (There are also van der wall interactions between the hydrophobic patch of the B6’-B7’ hairpin (L95E and L97E) and electrostatic interactions between K108 from the chain A loop between B7 and 8 and the 5’ phosphate)T6 forms a hydrogen bond with R64 of chain B, van der Waal interactions with L95E and L97E, and pi-pi interactions with W66 of chain B. The 5’ phosphate of T6 hydrogen bonds with the amino group of G134 of chain A. <ref>PMID: 23975200 </ref> | T4 stabilized by electrostatic interactions with R83 from chain A and E51 from chain A. T5 forms hydrogen bonds with K96 of Chain E and forms van der Waal contacts with K135 chain A. (There are also van der wall interactions between the hydrophobic patch of the B6’-B7’ hairpin (L95E and L97E) and electrostatic interactions between K108 from the chain A loop between B7 and 8 and the 5’ phosphate)T6 forms a hydrogen bond with R64 of chain B, van der Waal interactions with L95E and L97E, and pi-pi interactions with W66 of chain B. The 5’ phosphate of T6 hydrogen bonds with the amino group of G134 of chain A. <ref>PMID: 23975200 </ref> | ||
| - | [[Image:t4-6.jpg|300px|left|thumb|]] | ||
| Line 101: | Line 102: | ||
| - | [[Image:t7-8.jpg|300px|left|thumb|]]'''Nucleotides 7 and 8 (T7 and T8 respectively):''' Interact with Chains A2 and B2 | + | |
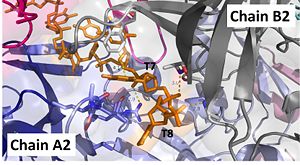
| + | [[Image:t7-8.jpg|300px|left|thumb|Interactions between DdrB and T7 and T8. (PDB: [[4HQB]]) This figure was generated using Pymol.]]'''Nucleotides 7 and 8 (T7 and T8 respectively):''' Interact with Chains A2 and B2 | ||
The 5’ phosphate of T7 hydrogen bonds with K94 of chain A and R132 of chain A. T7 forms pi-pi interactions with W66 of chain B and forms a hydrogen bond with K96. T8 is stabilized by a hydrophobic patch on the B6’-B7’ hairpin (V90 chain A. Phosphate groups of T8 stabilized through hydrogen bonding with A81, H80, and G106. <ref>PMID: 23975200 </ref> | The 5’ phosphate of T7 hydrogen bonds with K94 of chain A and R132 of chain A. T7 forms pi-pi interactions with W66 of chain B and forms a hydrogen bond with K96. T8 is stabilized by a hydrophobic patch on the B6’-B7’ hairpin (V90 chain A. Phosphate groups of T8 stabilized through hydrogen bonding with A81, H80, and G106. <ref>PMID: 23975200 </ref> | ||
| Line 109: | Line 111: | ||
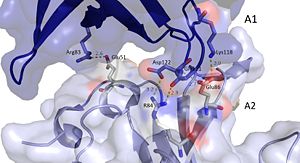
It should be noted that the quarternary structure enables the formation of a ssDNA channel. This structure forms independent of ssDNA binding and is stabilized by a variety of factors. The major interaction involve the interactions between the B6’-B7’ hairpin of chain E1 with the cleft from chains A2 and B2. Stabilization factors include electrostatic interactions and hydrogen bonding. Three essential salt bridges have also been identified in stabilizing this structure, they involve residues E51 and R83. <ref>PMID: 23975200 </ref> | It should be noted that the quarternary structure enables the formation of a ssDNA channel. This structure forms independent of ssDNA binding and is stabilized by a variety of factors. The major interaction involve the interactions between the B6’-B7’ hairpin of chain E1 with the cleft from chains A2 and B2. Stabilization factors include electrostatic interactions and hydrogen bonding. Three essential salt bridges have also been identified in stabilizing this structure, they involve residues E51 and R83. <ref>PMID: 23975200 </ref> | ||
| - | [[Image:E loop.jpg|300px|left|thumb|]] | + | [[Image:E loop.jpg|300px|left|thumb|The pentamer-pentamer interactions are stabilized by interactions involving the hairpin of chain E. (PDB:[[4HQB]]). This figure was generated using Pymol.]] |
| - | [[Image:A chains.jpg|300px|right|thumb|]] | + | [[Image:A chains.jpg|300px|right|thumb|The pentamer-pentamer interface is also stabilized by salt bridges and hydrogen bonds between the A chainds. (PDB:[[4HQB]]) This figure was generated using Pymol.]] |
| Line 148: | Line 150: | ||
It has been hypothesized that the most recent crystal structure of DdrB and ssDNA does not fully depict the ssDNA/DdrB interaction. <ref>PMID: 23975200 </ref> This idea is thought because the positively charged track (see above) on the surface of one side of the DdrB protein is not utilized in this crystal structure. It is possible that the DNA binds to this positive track and then proceed through the DNA channel formed by the pentamers. This is not unreasonable, as the crystal structure for uracil-DNA glycosylase failed to reveal an additional binding surface – that was later detected. Given the new-found role of DdrB in facilitating ssDNA strand annealing, it did not seem likely that the ssDNA channel revealed in the crystal structure could support this function. Therefore, DdrB mutants with an altered positive track were generated and tested. This experiment showed that the mutants could not bind ssDNA as well, suggesting that this surface area is involved in ssDNA binding. <ref>PMID: 23975200 </ref> | It has been hypothesized that the most recent crystal structure of DdrB and ssDNA does not fully depict the ssDNA/DdrB interaction. <ref>PMID: 23975200 </ref> This idea is thought because the positively charged track (see above) on the surface of one side of the DdrB protein is not utilized in this crystal structure. It is possible that the DNA binds to this positive track and then proceed through the DNA channel formed by the pentamers. This is not unreasonable, as the crystal structure for uracil-DNA glycosylase failed to reveal an additional binding surface – that was later detected. Given the new-found role of DdrB in facilitating ssDNA strand annealing, it did not seem likely that the ssDNA channel revealed in the crystal structure could support this function. Therefore, DdrB mutants with an altered positive track were generated and tested. This experiment showed that the mutants could not bind ssDNA as well, suggesting that this surface area is involved in ssDNA binding. <ref>PMID: 23975200 </ref> | ||
| - | [[Image:models.jpg|300px|left|thumb|]] | + | [[Image:models.jpg|300px|left|thumb|Potential Mechanisms for DdrB mediated ssDNA annealing as proposed by Sugiman-Marangos et al. (PDB: [[4HQB]] This figure was generated using Pymol.]] |
| Line 176: | Line 178: | ||
DdrB is unique to the genus Deinococcus and shares very few similarities with other ssDNA binding proteins. As mentioned above, many ssDNA binding proteins function through an OB fold. However, this fold is missing in DdrB and Ddrb present a novel ssDNA binding mechanism. | DdrB is unique to the genus Deinococcus and shares very few similarities with other ssDNA binding proteins. As mentioned above, many ssDNA binding proteins function through an OB fold. However, this fold is missing in DdrB and Ddrb present a novel ssDNA binding mechanism. | ||
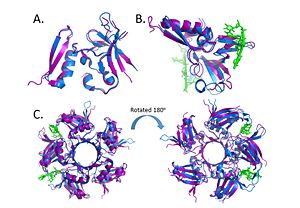
| - | Within the Deinococcus genus, DdrB is relatively conserved between species. The crystal structures of Deinococcus radiodurans and Deinococcus geothermalis are quite similar. However, there are some differences in the regions connecting B6-B7 and B7-B8. This difference may be due to difference in stability, the DNA present in the Deinococcus radiodurans structure may provide stabilize this part of the structure leading to the discrepancy.<ref>PMID: 23975200 </ref> [[Image:Alignment.jpg|300px|left|thumb|]] | + | Within the Deinococcus genus, DdrB is relatively conserved between species. The crystal structures of Deinococcus radiodurans and Deinococcus geothermalis are quite similar. However, there are some differences in the regions connecting B6-B7 and B7-B8. This difference may be due to difference in stability, the DNA present in the Deinococcus radiodurans structure may provide stabilize this part of the structure leading to the discrepancy.<ref>PMID: 23975200 </ref> [[Image:Alignment.jpg|300px|left|thumb| Alignment of the crystal structures of Deinococcus radiodurans and Deinococcus geothermalis. (A.) Alignment of chains c, (B.) Alignment of Chains a with DNA, (C.) Alignment of the pentamer. (PDB: [[4EXW]] and [[4HQB]]) This figure was generated using Pymol.]] |
there may also be similarities between DdrB and another Deinococcus DNA binding protein DdrA - however, more research is needed on the latter protein before any conclusions can be made. <ref>PMID: 23975200 </ref> | there may also be similarities between DdrB and another Deinococcus DNA binding protein DdrA - however, more research is needed on the latter protein before any conclusions can be made. <ref>PMID: 23975200 </ref> | ||
Revision as of 11:59, 30 April 2014
DdrB
| |||||||||||