|
A Brief Description:
DdrB is a single-stranded DNA binding protein that is found in bacteria from the genus Deinococcus. DdrB is thought to mediate the repair of genomic DNA following extensive genomic fragmentation induced by radiation and other agents. In this role, DdrB’s function has been compared to that of a unique stress-inducible equivalent of a single-stranded DNA binding protein. However, its function is not limited to DNA binding, as the protein has also been implicated in suppressing Rec J exonuclease activity [1] and promoting the annealing of complementary nucleotides [2].
General Description
DdrB is a 20kDa protein that is found in the bacterial genus Deinococcus. While it is not limited to Deinococcus radiodurans, its functional relevance has been clearly noted in this species. Deinococcus radiodurans is known for being a resilient bacteria that can withstand 15,000 Gy of γ radiation. This effectively shatters the genome into hundreds of 20-30kB fragments. [3] However, this bacteria is able to accurately repair its genome in a matter of hours and survive. This is an especially remarkable feat considering that the lethal dose of γ radiation in humans is 2-10 Gy. As a result, this finding has lead researchers to ask why Deinococcus radiodurans is able to survive such high levels of radiation? Radiation still induces many double stranded breaks (which are a prime contributor to lethality in other organisms [4]), so this ability has been attributed to several factors. One of these factors may include the genomic copy number as Deinococcus radiodurans contain 4-10 copies of their genome at any time.[5] However, the resistance to radiation-induced death has been primarily attributed to two components 1.) manganese dependent reactive oxygen species scavengers [6][7] and 2.) a unique repair pathway [8][9][10].
The repair pathway in Deinococcus radiodurans is thought to be a two step process. In the first part of repair, 5’ exonucleases generate long 3’ ssDNA extensions in the DNA fragments.[11] The second part of the repair process involves the extensions being put together through RecA mediated homologous recombination or through single-strand annealing.[12] These processes are also accompanied by an induction in the expression of a variety of proteins.[13] DdrB is one of the proteins that becomes highly upregulated following extensive DNA damage. This has been confirmed through evaluation of mRNA transcripts[14] and mass spectrometry proteomic analysis [15]. These studies revealed that DdrB is one of the top five upregulated genes following radiation exposure. To be specific, DdrB expression increases by over 40 fold following exposure to 3,000 Gy of γ radiation.[16] This is in sharp contrast to single stranded DNA binding proteins, which only have a minor increase in expression. For this and many other reasons, DdrB is thought to act as stress inducible equivalent of a single stranded DNA binding protein protecting and stabilizing any ssDNA that is present in the repair process.[17] [18] To further support the idea that DdrB has an essential role in the repair process of Deinococcus radiodurans, bacteria with no expression of DdrB experienced a 100 fold decrease in viability compared to the wild-type following exposure to 10,000 Gy of γ radiation. [19]
Subsequent studies have revealed that this protein is unique to the Deinococcus genus and varies in primary sequence length and composition depending upon the species. However, the protein structure and function remains similar. Electromobility shift assays have been used to further define DdrB function in terms of binding to nucleic acid.[20] These experiments have shown that DdrB preferentially binds to ssDNA with low uM affinity. [21] These studies also showed that DdrB has slight affinity for RNA and does not bind to double-stranded DNA. Even more recently, studies have shown that the function of DdrB is not limited to ssDNA binding. It has also been shown to promote the annealing of complementary oligonucleotides [22] and surprisingly has been shown to suppress RecJ exonuclease activity [23], further implicating DdrB in the repair of fragmented genomic DNA.
Structure Description
DdrB Monomers
Crystal Structures have revealed that DdrB is a mulitmeric protein composed of . The units contain 1 , 4 , 8 , 4 , 5 , and regions of undefined structure. [24] [25]
The N-terminal Domain
The N-terminal domain contains a . The is amphipathic, in which the hydrophobic regions pack toward features in the DdrB core.[26]
The DdrB Core
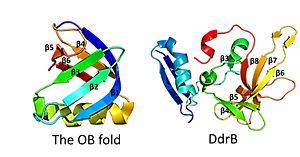 This figure highlights the differences between the classic OB fold found in single-stranded binding proteins and the novel structural features of DdrB. The OB fold is observed in the protein verotoxin-1, PDB code 2XSC . DdrB is modeled from the PDB structure 4HQB. This figure was generated using Pymol. This is followed by , which contain a solvent exposed face and another face that against the N-terminal motif. The beta sheets are anti-parallel and do not form an OB fold as determined by multiple servers iCOPS, DALI, 3D-BLAST, and MATRAS. [27] At the time of this finding (2010), the lack of an OB fold was surprising, since all ssDNA binding proteins were thought to bind to DNA through an OB fold. The OB fold is two three-stranded anti-parallel β sheets that form a five stranded β barrel. The differences between the DdrB beta strands and those in the OB fold include the topology of the β strands. DdrB B strands form an up and down topology and are not as angled as the strands in the OB fold. Furthermore, monomeric DdrB β strands do not form a beta barrel. Additionally, DdrB has different connectivity, no conserved glycine, and no β bulge. [28]
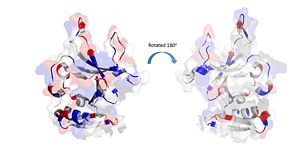 The electrostatic charges in the DdrB monomer. Blue represents positive charges, while red represents negative charges. (PDB: 4HQB). This figure was generated using Pymol. Positively Charged amino acids reside in the solvent exposed beta strands, which may potentially enable the binding of ssDNA.
that link beta sheet 6 to sheet 7 and beta sheet 7 to sheet 8 contain flexible regions with poor order as determined by limited to no density in the crystal structure. This finding suggests that these loops are intrinsically disordered.[29]
The C-terminal Domain
The C-terminal domain also contains regions with predicted intrinsic disorder.[30] The PSIpred server predicts that the last 35 residues of Deinococcus geothermalis are disordered. This prediction is supported by the solved crystal structure as the last 51 residues could not be determined. While, the structure of the C-terminal end is not known, it may still be of interest. A BLAST search revealed an 83 amino acid protein in Deinococcus geothermalis with 72% similarity and 62% identity to the disordered region of the C-terminus.[31] However, the function of this protein remains unknown. One hypothesis is that this region may mediate protein-protein interactions. Single stranded binding proteins also have disordered C-termini and contain negatively charged residues that mediate protein-protein interactions. As DdrB contains several conserved negatively charged residues it is thought that the C-terminus of this protein could also mediate protein-protein interactions.[32] However, this hypothesis may be debated as the C-terminus was not needed for radioresistance in Deinococcus radiodurans.[33]
DdrB Pentamer
The monomeric units of DdrB collectively form a with a 10 A pore in the center of this structure.[34] Other DNA binding proteins can thread DNA through a central pore, however this seems highly unlikely in the case of DdrB. The pore size appears too small (would need to be 14-40A) and has a net negative charge making it highly unfavorable for DNA interactions.[35] The beta-beta-alpha motif at the N-terminus of the monomer facilitates the formation of this as the beta sheets of the N-terminal beta-beta-alpha motif form a 10 stranded . [36]
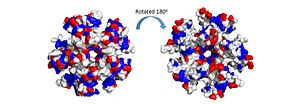 The electrostatic charges in the DdrB pentamer. Blue represents positive charges, while red represents negative charges. A positive “track” around the top of the pentamer may enable ssDNA binding to one side of the pentamer (left image). (PDB: 4HQB). This figure was generated using Pymol. The pentamer also contains a positive residue track on one side of the pentamer. These residues are thought to facilitate ssDNA binding and DdrB functionality. [37]
Structural features that relate to function
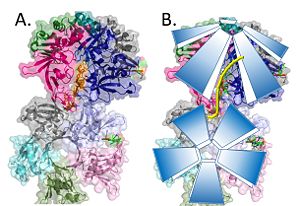 Model of the pentamer-pentamer structure. (PDB: 4HQB) This figure was generated using Pymol. Recently, a structure for Deinococcus radiodurans in complex with ssDNA has been solved.[38] The structure revealed that ssDNA bind in two clefts between three subunits. One 4 base pair strand of DT bound in the cleft between E and A, while the other 4 base pair strand of DT bound between A and B. Interestingly, when symmetry was applied to the crystal the dT strands appeared continuous. [39]
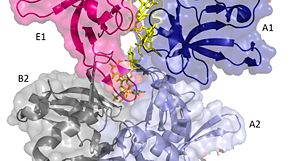 Two pentamers associate forming a channel for the ssDNA to thread through. (PDB: 4HQB) This figure was generated using Pymol. The crystal structure also determined that the pentameric ring binds to another pentameric ring. Given the continuous chain formed by the DT strands it appears that the chain passes through the channel formed by subunits A and E in one pentamer. (which will now be referred to as A1 and E1). To pass through the channel in the second pentamer between A and B (now A2 and B2). In this direction the DNA strand runs in the 5’ to 3’ direction.[40]
The interactions between DdrB and the DNA strand include:
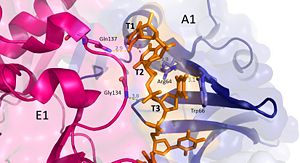 Interactions between DdrB and T1-T3. (PDB: 4HQB) This figure was generated using Pymol. Nucleotides 1, 2, 3 (T1, T2, and T3 respectively): Interact with Chains A1 and E1
T1 hydrogen bonds to Gln137 of chain E1. T1 and T2 base stack and form a cation pi interaction with R64 of the β3 strand in chain A1.
T3 hydrogen bonds to Arg64 of chain A1, forms a pi-pi interaction with Trp66 from chain A. T3 also forms hydrogen bonds with the 5’ phosphate and amino group of Gly134 in chain E1. [41]
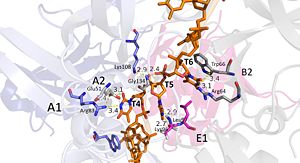 Interactions between DdrB and T4-T6. (PDB: 4HQB) This figure was generated using Pymol. Nucleotides 4, 5, 6 (T4, T5, and T6 respectively): Bridge the Pentamers
T4 stabilized by electrostatic interactions with Arg83 from chain A and Glu51 from chain A. T5 forms hydrogen bonds with Lys96 of Chain E and forms van der Waal contacts with Lys135 chain A. (There are also van der wall interactions between the hydrophobic patch of the B6’-B7’ hairpin (Leu95 and Leu97) and electrostatic interactions between Lys108 from the chain A loop between B7 and 8 and the 5’ phosphate)T6 forms a hydrogen bond with Arg64 of chain B, van der Waal interactions with Leu95 and Leu97, and pi-pi interactions with Trp66 of chain B. The 5’ phosphate of T6 hydrogen bonds with the amino group of Gly134 of chain A. [42]
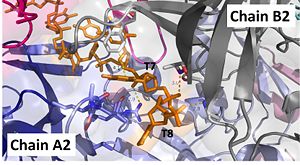 Interactions between DdrB and T7 and T8. (PDB: 4HQB) This figure was generated using Pymol. Interact with Chains A2 and B2
The 5’ phosphate of T7 hydrogen bonds with Lys94 of chain A and Arg132 of chain A. T7 forms pi-pi interactions with Trp66 of chain B and forms a hydrogen bond with Lys96. T8 is stabilized by a hydrophobic patch on the B6’-B7’ hairpin (Val90 chain A. Phosphate groups of T8 stabilized through hydrogen bonding with Ala81, His80, and Gly106. [43]
Interestingly the residues involved in DNA interactions are highly conserved among DdrB homologues. These binding interactions are not highly specific and contain a very small number of specific hydrogen bonds, suggesting that DdrB can bind to a variety of ssDNA strands.[44]
It should be noted that the quarternary structure enables the formation of a ssDNA channel. This structure forms independent of ssDNA binding and is stabilized by a variety of factors. The major interaction involve the interactions between the B6’-B7’ hairpin of chain E1 with the cleft from chains A2 and B2. Stabilization factors include electrostatic interactions and hydrogen bonding. Three essential salt bridges have also been identified in stabilizing this structure. Two key residues in these interactions are Glu51 and Arg83. [45]
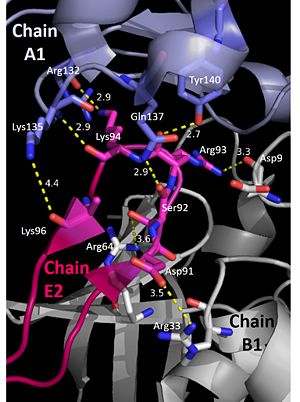 The pentamer-pentamer interactions are stabilized by interactions involving the hairpin of chain E. (PDB: 4HQB). This figure was generated using Pymol. 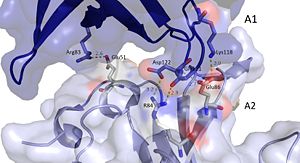 The pentamer-pentamer interface is also stabilized by salt bridges and hydrogen bonds between the A chainds. (PDB: 4HQB) This figure was generated using Pymol.
It has been hypothesized that the most recent crystal structure of DdrB and ssDNA does not fully depict the ssDNA/DdrB interaction. [46] This idea is thought because the positively charged track (see above) on the surface of one side of the DdrB protein is not utilized in this crystal structure. It is possible that the DNA binds to this positive track and then proceeds through the DNA channel formed by the pentamers. This is not unreasonable, as the crystal structure for uracil-DNA glycosylase failed to reveal an additional binding surface – that was later detected. [47] Given the new-found role of DdrB in facilitating ssDNA strand annealing, it did not seem likely that the ssDNA channel revealed in the crystal structure could support this function. Therefore, DdrB mutants were generated that altered the positive track and ssDNA binding was tested. This experiment showed that the mutants could not bind ssDNA as well, suggesting that this surface area is involved in ssDNA binding. [48]
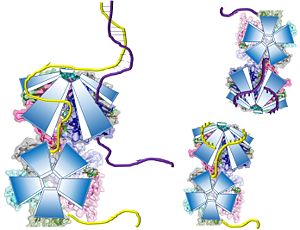 Potential Mechanisms for DdrB mediated ssDNA annealing as proposed by Sugiman-Marangos et al. (PDB: 4HQB This figure was generated using Pymol.
Related Proteins
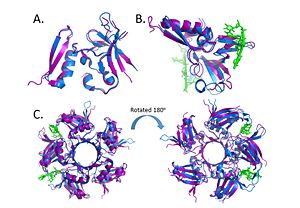 Alignment of the crystal structures of Deinococcus radiodurans and Deinococcus geothermalis. (A.) Alignment of chains c, (B.) Alignment of Chains a with DNA, (C.) Alignment of the pentamer. (PDB: 4EXW and 4HQB) This figure was generated using Pymol. DdrB is unique to the genus Deinococcus and shares very few similarities with other ssDNA binding proteins. As mentioned above, many ssDNA binding proteins function through an OB fold. However, this fold is missing in DdrB and Ddrb present a novel ssDNA binding mechanism.
Within the Deinococcus genus, DdrB is relatively conserved between species. A uniprot alignment between the primary structures of Deinococcus radiodurans and Deinococcus geothermalis showed that there was a 70% identity between the proteins. The crystal structures of Deinococcus radiodurans and Deinococcus geothermalis are also quite similar. However, there are some differences in the regions connecting B6-B7 and B7-B8. This difference may be due to difference in stability, the DNA present in the Deinococcus radiodurans structure may provide stabilize this part of the structure leading to the discrepancy.[49]
There may also be similarities between DdrB and another Deinococcus DNA binding protein DdrA - however, more research is needed on the latter protein before any conclusions can be made. [50]
Links to Available structures
4hqb,
4exw
References and Notes
- ↑ Jiao J, Wang L, Xia W, Li M, Sun H, Xu G, Tian B, Hua Y. Function and biochemical characterization of RecJ in Deinococcus radiodurans. DNA Repair (Amst). 2012 Apr 1;11(4):349-56. doi: 10.1016/j.dnarep.2011.11.008., Epub 2012 Jan 31. PMID:22301370 doi:http://dx.doi.org/10.1016/j.dnarep.2011.11.008
- ↑ Xu G, Lu H, Wang L, Chen H, Xu Z, Hu Y, Tian B, Hua Y. DdrB stimulates single-stranded DNA annealing and facilitates RecA-independent DNA repair in Deinococcus radiodurans. DNA Repair (Amst). 2010 Jul 1;9(7):805-12. doi: 10.1016/j.dnarep.2010.04.006., Epub 2010 May 6. PMID:20451472 doi:http://dx.doi.org/10.1016/j.dnarep.2010.04.006
- ↑ Zahradka K, Slade D, Bailone A, Sommer S, Averbeck D, Petranovic M, Lindner AB, Radman M. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature. 2006 Oct 5;443(7111):569-73. Epub 2006 Sep 27. PMID:17006450 doi:http://dx.doi.org/10.1038/nature05160
- ↑ Pardo B, Gomez-Gonzalez B, Aguilera A. DNA repair in mammalian cells: DNA double-strand break repair: how to fix a broken relationship. Cell Mol Life Sci. 2009 Mar;66(6):1039-56. doi: 10.1007/s00018-009-8740-3. PMID:19153654 doi:http://dx.doi.org/10.1007/s00018-009-8740-3
- ↑ Cox MM, Battista JR. Deinococcus radiodurans - the consummate survivor. Nat Rev Microbiol. 2005 Nov;3(11):882-92. PMID:16261171 doi:http://dx.doi.org/10.1038/nrmicro1264
- ↑ Zahradka K, Slade D, Bailone A, Sommer S, Averbeck D, Petranovic M, Lindner AB, Radman M. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature. 2006 Oct 5;443(7111):569-73. Epub 2006 Sep 27. PMID:17006450 doi:http://dx.doi.org/10.1038/nature05160
- ↑ Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004 Nov 5;306(5698):1025-8. Epub 2004 Sep 30. PMID:15459345 doi:http://dx.doi.org/10.1126/science.1103185
- ↑ Zahradka K, Slade D, Bailone A, Sommer S, Averbeck D, Petranovic M, Lindner AB, Radman M. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature. 2006 Oct 5;443(7111):569-73. Epub 2006 Sep 27. PMID:17006450 doi:http://dx.doi.org/10.1038/nature05160
- ↑ Slade D, Lindner AB, Paul G, Radman M. Recombination and replication in DNA repair of heavily irradiated Deinococcus radiodurans. Cell. 2009 Mar 20;136(6):1044-55. doi: 10.1016/j.cell.2009.01.018. PMID:19303848 doi:http://dx.doi.org/10.1016/j.cell.2009.01.018
- ↑ Tanaka M, Earl AM, Howell HA, Park MJ, Eisen JA, Peterson SN, Battista JR. Analysis of Deinococcus radiodurans's transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics. 2004 Sep;168(1):21-33. PMID:15454524 doi:http://dx.doi.org/10.1534/genetics.104.029249
- ↑ Slade D, Lindner AB, Paul G, Radman M. Recombination and replication in DNA repair of heavily irradiated Deinococcus radiodurans. Cell. 2009 Mar 20;136(6):1044-55. doi: 10.1016/j.cell.2009.01.018. PMID:19303848 doi:http://dx.doi.org/10.1016/j.cell.2009.01.018
- ↑ Slade D, Lindner AB, Paul G, Radman M. Recombination and replication in DNA repair of heavily irradiated Deinococcus radiodurans. Cell. 2009 Mar 20;136(6):1044-55. doi: 10.1016/j.cell.2009.01.018. PMID:19303848 doi:http://dx.doi.org/10.1016/j.cell.2009.01.018
- ↑ Dean CJ, Little JG, Serianni RW. The control of post irradiation DNA breakdown in Micrococcus radiodurans. Biochem Biophys Res Commun. 1970 Apr 8;39(1):126-34. PMID:5438290
- ↑ Liu Y, Zhou J, Omelchenko MV, Beliaev AS, Venkateswaran A, Stair J, Wu L, Thompson DK, Xu D, Rogozin IB, Gaidamakova EK, Zhai M, Makarova KS, Koonin EV, Daly MJ. Transcriptome dynamics of Deinococcus radiodurans recovering from ionizing radiation. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4191-6. Epub 2003 Mar 21. PMID:12651953 doi:http://dx.doi.org/10.1073/pnas.0630387100
- ↑ Basu B, Apte SK. Gamma radiation-induced proteome of Deinococcus radiodurans primarily targets DNA repair and oxidative stress alleviation. Mol Cell Proteomics. 2012 Jan;11(1):M111.011734. doi: 10.1074/mcp.M111.011734., Epub 2011 Oct 11. PMID:21989019 doi:http://dx.doi.org/10.1074/mcp.M111.011734
- ↑ Tanaka M, Earl AM, Howell HA, Park MJ, Eisen JA, Peterson SN, Battista JR. Analysis of Deinococcus radiodurans's transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics. 2004 Sep;168(1):21-33. PMID:15454524 doi:http://dx.doi.org/10.1534/genetics.104.029249
- ↑ Tanaka M, Earl AM, Howell HA, Park MJ, Eisen JA, Peterson SN, Battista JR. Analysis of Deinococcus radiodurans's transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics. 2004 Sep;168(1):21-33. PMID:15454524 doi:http://dx.doi.org/10.1534/genetics.104.029249
- ↑ Norais CA, Chitteni-Pattu S, Wood EA, Inman RB, Cox MM. DdrB protein, an alternative Deinococcus radiodurans SSB induced by ionizing radiation. J Biol Chem. 2009 Aug 7;284(32):21402-11. doi: 10.1074/jbc.M109.010454. Epub 2009, Jun 10. PMID:19515845 doi:http://dx.doi.org/10.1074/jbc.M109.010454
- ↑ Tanaka M, Earl AM, Howell HA, Park MJ, Eisen JA, Peterson SN, Battista JR. Analysis of Deinococcus radiodurans's transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics. 2004 Sep;168(1):21-33. PMID:15454524 doi:http://dx.doi.org/10.1534/genetics.104.029249
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Xu G, Lu H, Wang L, Chen H, Xu Z, Hu Y, Tian B, Hua Y. DdrB stimulates single-stranded DNA annealing and facilitates RecA-independent DNA repair in Deinococcus radiodurans. DNA Repair (Amst). 2010 Jul 1;9(7):805-12. doi: 10.1016/j.dnarep.2010.04.006., Epub 2010 May 6. PMID:20451472 doi:http://dx.doi.org/10.1016/j.dnarep.2010.04.006
- ↑ Jiao J, Wang L, Xia W, Li M, Sun H, Xu G, Tian B, Hua Y. Function and biochemical characterization of RecJ in Deinococcus radiodurans. DNA Repair (Amst). 2012 Apr 1;11(4):349-56. doi: 10.1016/j.dnarep.2011.11.008., Epub 2012 Jan 31. PMID:22301370 doi:http://dx.doi.org/10.1016/j.dnarep.2011.11.008
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Bouthier de la Tour C, Boisnard S, Norais C, Toueille M, Bentchikou E, Vannier F, Cox MM, Sommer S, Servant P. The deinococcal DdrB protein is involved in an early step of DNA double strand break repair and in plasmid transformation through its single-strand annealing activity. DNA Repair (Amst). 2011 Dec 10;10(12):1223-31. doi: 10.1016/j.dnarep.2011.09.010., Epub 2011 Oct 2. PMID:21968057 doi:http://dx.doi.org/10.1016/j.dnarep.2011.09.010
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010 Jun;38(10):3432-40. Epub 2010 Feb 2. PMID:20129942 doi:10.1093/nar/gkq036
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Roberts VA, Pique ME, Hsu S, Li S, Slupphaug G, Rambo RP, Jamison JW, Liu T, Lee JH, Tainer JA, Ten Eyck LF, Woods VL Jr. Combining H/D exchange mass spectroscopy and computational docking reveals extended DNA-binding surface on uracil-DNA glycosylase. Nucleic Acids Res. 2012 Jul;40(13):6070-81. Epub 2012 Apr 6. PMID:22492624 doi:http://dx.doi.org/10.1093/nar/gks291
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
- ↑ Sugiman-Marangos SN, Peel JK, Weiss YM, Ghirlando R, Junop MS. Crystal structure of the DdrB/ssDNA complex from Deinococcus radiodurans reveals a DNA binding surface involving higher-order oligomeric states. Nucleic Acids Res. 2013 Aug 23. PMID:23975200 doi:10.1093/nar/gkt759
|












