We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 930
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
Myosin is a large asymmetric molecule with a MW of about 500,000 kDa. It consist of two globular head domains termed myosin subfragment 1 (S1), one neck subfragment 2 (S2) and a light meromyosin tail (LMM) <ref>PMID: 8203020</ref>. Myosin S1 unit comprises of a motor domain (MD) and a lever arm (Fig.3). By 2000 the structures of three scallop myosin S1 isoforms have been determined <ref>PMID: 11016966</ref><ref>PMID: 10338210</ref>, which are: | Myosin is a large asymmetric molecule with a MW of about 500,000 kDa. It consist of two globular head domains termed myosin subfragment 1 (S1), one neck subfragment 2 (S2) and a light meromyosin tail (LMM) <ref>PMID: 8203020</ref>. Myosin S1 unit comprises of a motor domain (MD) and a lever arm (Fig.3). By 2000 the structures of three scallop myosin S1 isoforms have been determined <ref>PMID: 11016966</ref><ref>PMID: 10338210</ref>, which are: | ||
| - | • S1 nucleotide-free state corresponding to the rigor state of myosin, actin complex | + | • S1 nucleotide-free state corresponding to the rigor state of myosin, actin complex [http://www.pdb.org/pdb/explore/explore.do?structureId=1DFK 1DFK] |
• S1 Mg-ADP.VO4 state corresponding to the transition state (1kk8) | • S1 Mg-ADP.VO4 state corresponding to the transition state (1kk8) | ||
| Line 35: | Line 35: | ||
==The subdomains of the motor domain== | ==The subdomains of the motor domain== | ||
| - | [[Image:SUBDOMAINS.gif|300px|right|thumb|Figure 3. The | + | [[Image:SUBDOMAINS.gif|300px|right|thumb|Figure 3. The subdomains on myosin S1 unit] |
The MD of S1 unit is most frequently described as consisting of 4 subdomains: the converter, the N-terminal subdomain, and upper and lower 50-kDa subdomains <ref>PMID: 10338210</ref>. They are linked together by 3 single-stranded joints termed the switch II (residue IIe-461 to Asn-470), the relay (residues Asn-489 to ASP-519), and SH1 helix (Cys-693 to Phe-707)(Fig. 3) <ref>PMID: 11016966</ref>. | The MD of S1 unit is most frequently described as consisting of 4 subdomains: the converter, the N-terminal subdomain, and upper and lower 50-kDa subdomains <ref>PMID: 10338210</ref>. They are linked together by 3 single-stranded joints termed the switch II (residue IIe-461 to Asn-470), the relay (residues Asn-489 to ASP-519), and SH1 helix (Cys-693 to Phe-707)(Fig. 3) <ref>PMID: 11016966</ref>. | ||
Revision as of 11:54, 16 May 2014
| This Sandbox is Reserved from 01/04/2014, through 30/06/2014 for use in the course "510042. Protein structure, function and folding" taught by Prof Adrian Goldman, Tommi Kajander, Taru Meri, Konstantin Kogan and Juho Kellosalo at the University of Helsinki. This reservation includes Sandbox Reserved 923 through Sandbox Reserved 947. |
To get started:
More help: Help:Editing |
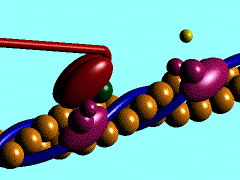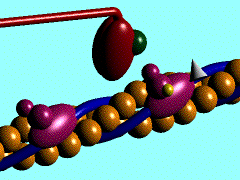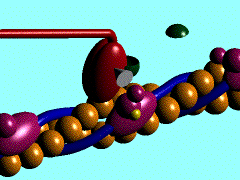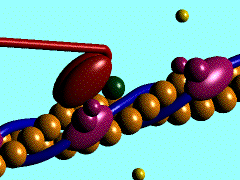
Scallop myosin head in its pre power stroke state
Introduction
In the striated muscle the actin and myosin proteins form ordered basic units called sarcomeres. Muscle contraction is achieved by the mechanical sliding of myosin filament (thick filament) along the actin filament (thin filament), Fig. 1. The major constituent of the myosin filament is myosin, a motor protein responsible for converting chemical energy to mechanical movement. In the presence of Ca2+ and Mg2+, myosin is able to cyclically bind ATP and hydrolyse it to ADP + Pi , triggering subsequent myosin-actin detachment, reattachment and power stroke, so called contractile reaction (Fig.2).
.
Introduction of the Myosin head S1
| |||||||||||