This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 930
From Proteopedia
| Line 64: | Line 64: | ||
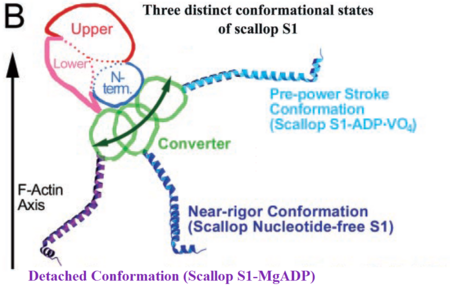
The MD has different conformational states in each step of the contractile cycle. The conformation of the MD in each state depends on which nucleotide is bound to the active site (if any). In each structural state the conformation of the MD changes relatively little, but these changes are enough to cause a substantial difference in the position of the lever arm (Fig. 4) <ref>PMID: 11016966</ref>. | The MD has different conformational states in each step of the contractile cycle. The conformation of the MD in each state depends on which nucleotide is bound to the active site (if any). In each structural state the conformation of the MD changes relatively little, but these changes are enough to cause a substantial difference in the position of the lever arm (Fig. 4) <ref>PMID: 11016966</ref>. | ||
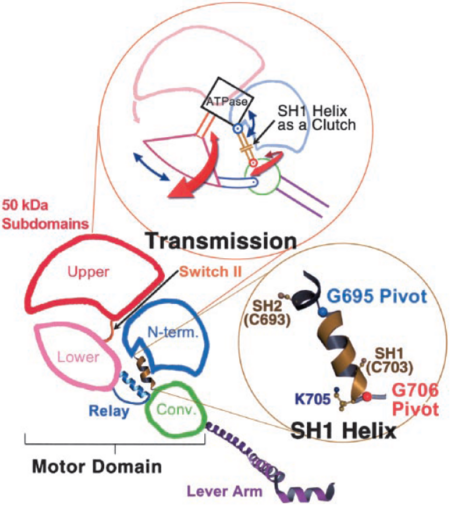
| - | The 50-kDa upper and lower subdomains as well as the converter control the motor function of the myosin head by rotating around the N-terminal subdomain. The rotations depend on the conformational changes of the 3 joints; switch II, SH1 helix region, and relay. <ref>PMID: 15184651</ref> The joints work together in the transition between the different conformational states of MD to control the overall organization of the myosin head. They also allow communication between the nucleotide-bonding pocket, acting-binding interface and the lever arm <ref>PMID: 11016966</ref>. | + | [[Image:Myosin turn.png|450px|right|thumb| Figure 5. Arrows in the upper figure show the direction of rotation of the 50-kDa lower and upper subdomains and the converter around the N-terminal subdomain. Lower picture shows the pivots around which SH1 unwinds. (Himmel 2002)]] |
| + | The 50-kDa upper and lower subdomains as well as the converter control the motor function of the myosin head by rotating around the N-terminal subdomain (Fig 5). The rotations depend on the conformational changes of the 3 joints; switch II, SH1 helix region, and relay. <ref>PMID: 15184651</ref> The joints work together in the transition between the different conformational states of MD to control the overall organization of the myosin head. They also allow communication between the nucleotide-bonding pocket, acting-binding interface and the lever arm <ref>PMID: 11016966</ref>. | ||
Switch II, a catalytic loop of the nucleotide-binding pocket, moves in and out of the nucleotide-binding pocket during enzymatic activity. It is responsible for the unwinding of SH1 helix, along with the conformational changes caused by nucleotide binding. Upon unwinding helix SH1 uncouples the converter/lever module from the MD. <ref>PMID: 12297624</ref> Movement of the converter is controlled by the relay joint. The converter/relay module attains different conformations changing the position of the lever arm and thus giving rise to the different states of the actomyosin cycle <ref>PMID: 11016966</ref>. | Switch II, a catalytic loop of the nucleotide-binding pocket, moves in and out of the nucleotide-binding pocket during enzymatic activity. It is responsible for the unwinding of SH1 helix, along with the conformational changes caused by nucleotide binding. Upon unwinding helix SH1 uncouples the converter/lever module from the MD. <ref>PMID: 12297624</ref> Movement of the converter is controlled by the relay joint. The converter/relay module attains different conformations changing the position of the lever arm and thus giving rise to the different states of the actomyosin cycle <ref>PMID: 11016966</ref>. | ||
Revision as of 10:52, 17 May 2014
| This Sandbox is Reserved from 01/04/2014, through 30/06/2014 for use in the course "510042. Protein structure, function and folding" taught by Prof Adrian Goldman, Tommi Kajander, Taru Meri, Konstantin Kogan and Juho Kellosalo at the University of Helsinki. This reservation includes Sandbox Reserved 923 through Sandbox Reserved 947. |
To get started:
More help: Help:Editing |
Contents |
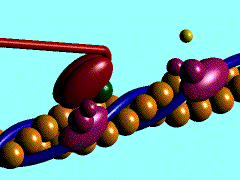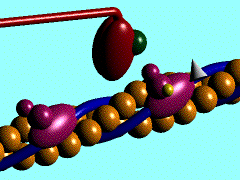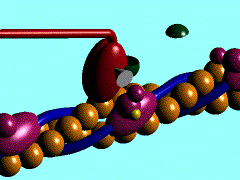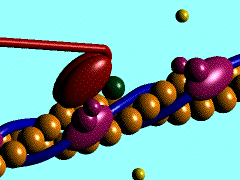
Scallop myosin head in its pre power stroke state
Introduction
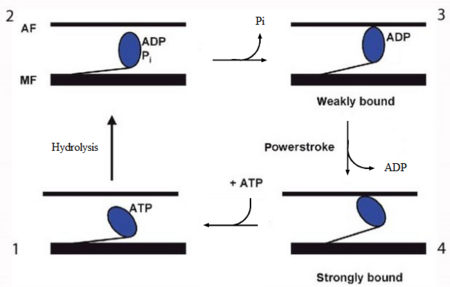
In the striated muscle the actin and myosin proteins form ordered basic units called sarcomeres. Muscle contraction is achieved by the mechanical sliding of myosin filament (thick filament) along the actin filament (thin filament), Fig. 1. The major constituent of the myosin filament is myosin, a motor protein responsible for converting chemical energy to mechanical movement. In the presence of Ca2+ and Mg2+, myosin is able to cyclically bind ATP and hydrolyse it to ADP + Pi , triggering subsequent myosin-actin detachment, reattachment and power stroke, so called contractile reaction (Fig.2).
.
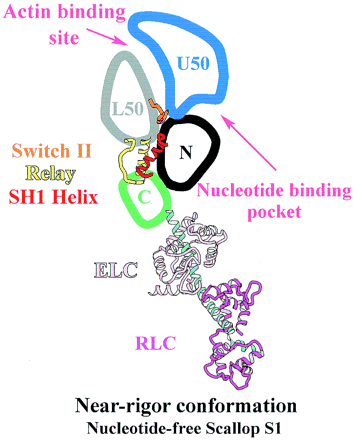
Introduction of the Myosin head S1
| |||||||||||
References
- ↑ Rayment I, Holden HM. The three-dimensional structure of a molecular motor. Trends Biochem Sci. 1994 Mar;19(3):129-34. PMID:8203020
- ↑ Houdusse A, Szent-Gyorgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11238-43. PMID:11016966 doi:10.1073/pnas.200376897
- ↑ Houdusse A, Kalabokis VN, Himmel D, Szent-Gyorgyi AG, Cohen C. Atomic structure of scallop myosin subfragment S1 complexed with MgADP: a novel conformation of the myosin head. Cell. 1999 May 14;97(4):459-70. PMID:10338210
- ↑ Houdusse A, Kalabokis VN, Himmel D, Szent-Gyorgyi AG, Cohen C. Atomic structure of scallop myosin subfragment S1 complexed with MgADP: a novel conformation of the myosin head. Cell. 1999 May 14;97(4):459-70. PMID:10338210
- ↑ Houdusse A, Szent-Gyorgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11238-43. PMID:11016966 doi:10.1073/pnas.200376897
- ↑ Houdusse A, Szent-Gyorgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11238-43. PMID:11016966 doi:10.1073/pnas.200376897
- ↑ Risal D, Gourinath S, Himmel DM, Szent-Gyorgyi AG, Cohen C. Myosin subfragment 1 structures reveal a partially bound nucleotide and a complex salt bridge that helps couple nucleotide and actin binding. Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):8930-5. Epub 2004 Jun 7. PMID:15184651 doi:10.1073/pnas.0403002101
- ↑ Houdusse A, Szent-Gyorgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11238-43. PMID:11016966 doi:10.1073/pnas.200376897
- ↑ Risal D, Gourinath S, Himmel DM, Szent-Gyorgyi AG, Cohen C. Myosin subfragment 1 structures reveal a partially bound nucleotide and a complex salt bridge that helps couple nucleotide and actin binding. Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):8930-5. Epub 2004 Jun 7. PMID:15184651 doi:10.1073/pnas.0403002101
- ↑ Houdusse A, Szent-Gyorgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11238-43. PMID:11016966 doi:10.1073/pnas.200376897
- ↑ Himmel DM, Gourinath S, Reshetnikova L, Shen Y, Szent-Gyorgyi AG, Cohen C. Crystallographic findings on the internally uncoupled and near-rigor states of myosin: further insights into the mechanics of the motor. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):12645-50. Epub 2002 Sep 24. PMID:12297624 doi:10.1073/pnas.202476799
- ↑ Houdusse A, Szent-Gyorgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11238-43. PMID:11016966 doi:10.1073/pnas.200376897
- ↑ Houdusse A, Szent-Gyorgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11238-43. PMID:11016966 doi:10.1073/pnas.200376897
- ↑ Himmel DM, Gourinath S, Reshetnikova L, Shen Y, Szent-Gyorgyi AG, Cohen C. Crystallographic findings on the internally uncoupled and near-rigor states of myosin: further insights into the mechanics of the motor. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):12645-50. Epub 2002 Sep 24. PMID:12297624 doi:10.1073/pnas.202476799
- ↑ Himmel DM, Gourinath S, Reshetnikova L, Shen Y, Szent-Gyorgyi AG, Cohen C. Crystallographic findings on the internally uncoupled and near-rigor states of myosin: further insights into the mechanics of the motor. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):12645-50. Epub 2002 Sep 24. PMID:12297624 doi:10.1073/pnas.202476799
- ↑ Risal D, Gourinath S, Himmel DM, Szent-Gyorgyi AG, Cohen C. Myosin subfragment 1 structures reveal a partially bound nucleotide and a complex salt bridge that helps couple nucleotide and actin binding. Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):8930-5. Epub 2004 Jun 7. PMID:15184651 doi:10.1073/pnas.0403002101
- ↑ Himmel DM, Gourinath S, Reshetnikova L, Shen Y, Szent-Gyorgyi AG, Cohen C. Crystallographic findings on the internally uncoupled and near-rigor states of myosin: further insights into the mechanics of the motor. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):12645-50. Epub 2002 Sep 24. PMID:12297624 doi:10.1073/pnas.202476799