Urease
From Proteopedia
| Line 70: | Line 70: | ||
considers UreABC as a functional unit. Studies of ''Klebsiella aerogenes'' urease activation pathway revealed that three accessory proteins – <span style="color:yellow;background-color:black;font-weight:bold;">UreD (yellow)</span>, <span style="color:cyan;background-color:black;font-weight:bold;">UreF (cyan)</span>, <font color='magenta'><b>UreG (magenta)</b></font> – are essential for the production of a functional urease. These proteins sequentially bind to form the <scene name='Journal:JBSD:6/Cv/8'>(UreABC-UreD)3</scene>, <scene name='Journal:JBSD:6/Cv/9'>(UreABC-UreDF)3</scene>, and <scene name='Journal:JBSD:6/Cv/10'>(UreABC-UreDFG)3</scene> activation complexes. <scene name='Journal:JBSD:6/Cv/12'>Click here to see this structure</scene> is rotated by 90º. In this work we submitted structural models of such proteins to macromolecular docking calculations with ''K. aerogenes'' urease, which lead to a putative structure for the urease activation complex. | considers UreABC as a functional unit. Studies of ''Klebsiella aerogenes'' urease activation pathway revealed that three accessory proteins – <span style="color:yellow;background-color:black;font-weight:bold;">UreD (yellow)</span>, <span style="color:cyan;background-color:black;font-weight:bold;">UreF (cyan)</span>, <font color='magenta'><b>UreG (magenta)</b></font> – are essential for the production of a functional urease. These proteins sequentially bind to form the <scene name='Journal:JBSD:6/Cv/8'>(UreABC-UreD)3</scene>, <scene name='Journal:JBSD:6/Cv/9'>(UreABC-UreDF)3</scene>, and <scene name='Journal:JBSD:6/Cv/10'>(UreABC-UreDFG)3</scene> activation complexes. <scene name='Journal:JBSD:6/Cv/12'>Click here to see this structure</scene> is rotated by 90º. In this work we submitted structural models of such proteins to macromolecular docking calculations with ''K. aerogenes'' urease, which lead to a putative structure for the urease activation complex. | ||
The presented model for this complex is the first to include UreG and to use the current data on the activation pathway to guide the docking calculations. Despite the urease activation process being far more complex, our results are likely to expand the current knowledge on this essential step for proper ureolytic activity, aiding further high resolution studies of this macromolecular assembly by providing a 3D scaffold to work upon. | The presented model for this complex is the first to include UreG and to use the current data on the activation pathway to guide the docking calculations. Despite the urease activation process being far more complex, our results are likely to expand the current knowledge on this essential step for proper ureolytic activity, aiding further high resolution studies of this macromolecular assembly by providing a 3D scaffold to work upon. | ||
| + | |||
| + | === Fluoride inhibition of Sporosarcina pasteurii urease: structure and thermodynamics === | ||
| + | <big>Stefano Benini, Michele Cianci, Luca Mazzei and Stefano Ciurli</big> <ref>PMID 25113581 </ref> | ||
| + | <hr/> | ||
| + | <b>Molecular Tour</b><br> | ||
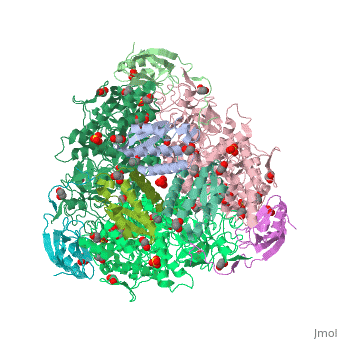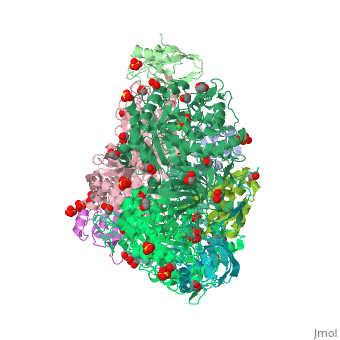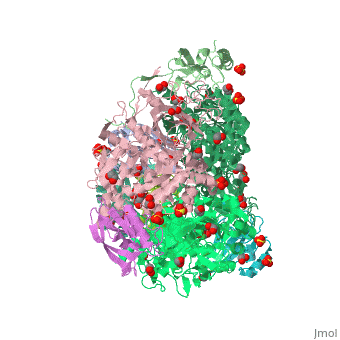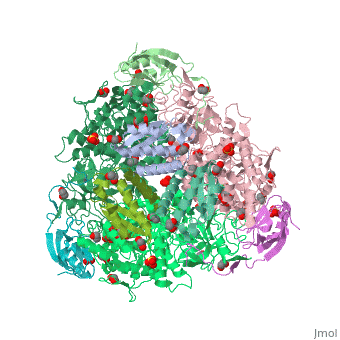
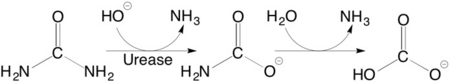
| + | <scene name='59/596313/Cv/3'>Urease</scene> (it is in homotrimeric form of αβγ heterotrimer) is a nickel-dependent enzyme (<span style="color:green;background-color:black;font-weight:bold;">Ni(II) ions are shown as green spheres</span>, <font color='darkmagenta'><b>α</b></font>, <span style="color:yellow;background-color:black;font-weight:bold;">β</span>, and <span style="color:deeppink;background-color:black;font-weight:bold;">γ</span> subunits are colored in <font color='darkmagenta'><b>darkmagenta</b></font>, <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span>, <span style="color:deeppink;background-color:black;font-weight:bold;">deeppink</span>, respectively) and a virulence factor for ureolytic bacterial human pathogens, but it is also necessary to convert urea (see static image below), the most worldwide used fertiliser, into forms of nitrogen that can be taken up by crop plants. | ||
| + | [[Image:Scheme_1.png|left|450px|thumb|]] | ||
| + | {{Clear}} | ||
| + | A strategy to control the activity of urease for medical and agricultural applications is to use enzyme inhibitors. Fluoride is a known urease inhibitor, but the structural basis of its mode of inhibition are still undetermined. Here, kinetic studies on the fluoride-induced inhibition of urease from ''Sporosarcina pasteurii'', a widespread and highly ureolytic soil bacterium, revealed a mixed competitive and uncompetitive mechanism. The pH-dependence of the inhibition constants, investigated in the 6.5-8.0 range, reveals a predominant uncompetitive mechanism that increases by increasing the pH, and a lesser competitive inhibition that increases by lowering the pH. Ten crystal structures of the enzyme were independently determined using five crystals of the <scene name='59/596313/Cv/13'>native form</scene> and five crystals of the protein crystallised in the presence of fluoride. The analysis of these structures revealed the presence of <scene name='59/596313/Cv/14'>two fluoride anions coordinated to the Ni(II) ions in the active site</scene>, in terminal and bridging positions (<span style="color:gold;background-color:black;font-weight:bold;">both fluorides are colored in gold</span>). <scene name='59/596313/Cv/20'>Click here to see animation</scene>. | ||
| + | |||
| + | Structural studies on ureases have revealed that the immediate environment around the two Ni(II) ions at the active site is conserved, as to induce a common mechanism of catalysis whose key step is the nucleophilic attack of the nickel-bridging hydroxide on the urea molecule bound to the bimetallic nickel cluster via O and N atoms (see static image below). | ||
| + | [[Image:Scheme_2.png|left|450px|thumb|]] | ||
| + | {{Clear}} | ||
| + | The present study consistently supports an interaction of fluoride with the nickel centres in the urease active site in which <scene name='59/596313/Cv/17'>one fluoride competitively binds</scene> (<span style="color:salmon;background-color:black;font-weight:bold;">colored in salmon</span>) to the Ni(II) ion proposed to coordinate urea in the initial step of the catalytic mechanism, while <scene name='59/596313/Cv/18'>another fluoride uncompetitively substitutes</scene> (<span style="color:cyan;background-color:black;font-weight:bold;">colored in cyan</span>) the Ni(II)-bridging hydroxide, blocking its nucleophilic attack on urea. | ||
</StructureSection> | </StructureSection> | ||
__NOTOC__ | __NOTOC__ | ||
Revision as of 11:20, 2 October 2014
| |||||||||||
3D structures of urease
Updated on 02-October-2014
2kau, 1kra, 1fwj, 1ejx, 1ejw, 4ep8 – KaUA α+β+γ chains – Klebsiella aerogenes
1ef2 - KaUA α+β+γ chains Mn substituted
1krb, 1krc, 1fwa, 1fwb, 1fwc, 1fwd, 1fwf , 1fwg, 1fwh, 1fwi – KaUA α (mutant) +β (mutant) +γ (mutant) chains
1a5k, 1a5l, 1a5m, 1ejr, 1ejs, 1ejt, 1eju, 1ejv - KaUA α+β+γ (mutant) chains
2ubp - BpUA α+β+γ chains – Bacillus pasteurii
1e9z - HpUA α+β chains – Helicobacter pylori
3qga, 3qgk - UA β/γ chains Fe containing – Helicobacter mustelae
2fvh - UA γ chain – Mycobacterium tuberculosis
3la4 – UA – horse bean
4epb, 4epd, 4epe - UA α+β+γ chains – Enterobacter aerogenes
4ac7 - UA α+β+γ chains – Sporosarcina pasteurii
4fur - UA γ2 chain – Enterobacter melitensis
4g7e - UA – pigeon pea
4gy7 - jbUA – jack bean
Urease binary complex
1a5n, 1a5o - KaUA α+β+γ (mutant) chains + formate
1fwe – KaUA α (mutant) +β (mutant) +γ (mutant) chains + acetohydroxamic acid
1ubp - BpUA α+β+γ chains + mercaptoethanol
3ubp - BpUA α+β+γ chains + diamidophosphate
4ubp - BpUA α+β+γ chains + acetohydroxamic acid
1ie7 - BpUA α+β+γ chains + phosphate
1s3t - BpUA α+β+γ chains + borate
1e9y - HpUA α+β chains + acetohydroxamic acid
4goa - jbUA + F
4h9m - jbUA + acetohydroxamic acid
Additional Resources
For additional information on Urinary Tract Infection, See: 1tr7
For additional information on Helicobacter Pylori, See: 1e9z
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 PMID: PMC2443974
- ↑ http://www.jbc.org/content/277/35/e23.full?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&searchid=1130442887043_7599&stored_search=&FIRSTINDEX=60&tocsectionid=Classics&sortspec=PUBDATE_SORTDATE+desc
- ↑ Andrews, R. K., Blakeley, R. L. & Zerner, B. (1984). Urea and urease. Adv. Inorg. Biochem. 6, 245–283.
- ↑ Dixon, N. E., Riddles, P. W., Gazzola, C., Blakeley, R. L. & Zerner, B. (1980). Jack been urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the “abnormal” ultraviolet spectrum. The nickel content of jack beans. Can. J. Biochem. 58, 474–480.
- ↑ Moncrief, M. C. & Hausinger, R. P. (1996). Nickel incorporation into urease. In Mechanisms of Metallo- center Assembly (Hausinger, R. P., Eichhorn, G. L. & Marzilli, L. G., eds), pp. 151–171, Elsevier Press, New York, NY.
- ↑ 6.0 6.1 Covacci, A., Telford, J. L., Del Giudice, G., Parsonnet, J. & Rappuoli, R. (1999). Helicobacter pylori virulence and genetic geography. Science, 284, 1328–1333.
- ↑ Polacco, J. C. & Holland, M. A. (1993). Roles of urease in plant cells. Int. Rev. Cytol. 145, 65–103.
- ↑ 8.0 8.1 http://en.wikipedia.org/wiki/Urease
- ↑ 9.0 9.1 9.2 Mobley, H. L. T., Island, M. D. & Hausinger, R. P. (1995). Molecular biology of microbial ureases. Microbiol. Rev. 59, 451–480.
- ↑ http://www.cell.com/structure/abstract/S0969-2126(99)80026-4#.
- ↑ Cicmanec JF, Helmers SL, Evans AT. Office practice survey of urease positive bacterial pathogens causing urinary tract infections. Urology. 1980 Sep;16(3):274-6. PMID:6999699
- ↑ Dixon, N. E., Riddles, P. W., Gazzola, C., Blakeley, R. L. & Zerner, B. (1980). Jack been urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the “abnormal” ultraviolet spectrum. The nickel content of jack beans. Can. J. Biochem. 58, 474–480.
- ↑ Becker-Ritt, A. B., Martinelli, A. H. S., Mitidieri, S., Feder, V., Wassermann, G. E., Santi, L. et al. (2007). Antifungal activity of plant and bacterial ureases. Toxicon, 50, 971–983.
- ↑ 14.0 14.1 Follmer, C., Real-Guerra, R., Wassermann, G. E., Olivera-Severo, D. & Carlini, C. R. (2004). Jackbean, soybean and Bacillus pasteurii ureases—biological effects unrelated to ureolytic activity. Eur. J. Biochem. 271, 1357–1363.
- ↑ Karplus, P. A., Pearson, M. A. & Hausinger, R. P. (1997). 70 years of crystalline urease: what have we learnt? Acc. Chem. Res. 30, 330–337.
- ↑ Benini, S., Rypneiwski, W. R., Wilson, K. S., Meletti, S., Ciurli, S. & Mangani, S. (1999). A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Structure, 7, 205–216.
- ↑ 17.0 17.1 http://tonga.usip.edu/jsnow/chem348/recitation8.pdf
- ↑ http://emedicine.medscape.com/article/1174503-overview
- ↑ http://www.nucdf.org/ucd_treatment.htm
- ↑ Benini S, Kosikowska P, Cianci M, Mazzei L, Vara AG, Berlicki L, Ciurli S. The crystal structure of Sporosarcina pasteurii urease in a complex with citrate provides new hints for inhibitor design. J Biol Inorg Chem. 2013 Mar;18(3):391-9. doi: 10.1007/s00775-013-0983-7. Epub, 2013 Feb 15. PMID:23412551 doi:10.1007/s00775-013-0983-7
- ↑ Kcx - Lysine NZ-carboxylic acid
- ↑ Zambelli B, Banaszak K, Merloni A, Kiliszek A, Rypniewski W, Ciurli S. Selectivity of Ni(II) and Zn(II) binding to Sporosarcina pasteurii UreE, a metallochaperone in the urease assembly: a calorimetric and crystallographic study. J Biol Inorg Chem. 2013 Dec;18(8):1005-17. doi: 10.1007/s00775-013-1049-6. Epub, 2013 Oct 15. PMID:24126709 doi:http://dx.doi.org/10.1007/s00775-013-1049-6
- ↑ Ligabue-Braun R, Real-Guerra R, Carlini CR, Verli H. Evidence-based docking of the urease activation complex. J Biomol Struct Dyn. 2012 Sep 10. PMID:22962938 doi:10.1080/07391102.2012.713782
- ↑ Benini S, Cianci M, Mazzei L, Ciurli S. Fluoride inhibition of Sporosarcina pasteurii urease: structure and thermodynamics. J Biol Inorg Chem. 2014 Aug 12. PMID:25113581 doi:http://dx.doi.org/10.1007/s00775-014-1182-x
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Andrea Graydon, Alexander Berchansky, David Canner, OCA