Adrenergic receptor
From Proteopedia
| Line 44: | Line 44: | ||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | + | *α-2 adrenergic receptor | |
| - | [[1ho9]], [[1hod]] – hA2AR peptide (mutant) – human - NMR<br /> | + | **[[1ho9]], [[1hod]] – hA2AR peptide (mutant) – human - NMR<br /> |
| - | [[1hof]], [[1hll]] - hA2AR peptide – NMR<br /> | + | **[[1hof]], [[1hll]] - hA2AR peptide – NMR<br /> |
| - | + | *β-1 adrenergic receptor | |
| - | [[2y01]], [[4gpo]] – tB1AR fragment (mutant) – turkey<br /> | + | **[[2y01]], [[4gpo]] – tB1AR fragment (mutant) – turkey<br /> |
| - | [[1dep]] – tB1AR peptide - NMR<br /> | + | **[[1dep]] – tB1AR peptide - NMR<br /> |
| - | [[2y00]], [[2y02]], [[2y03]], [[2y04]], [[2vt4]], [[4ami]], [[4amj]] - tB1AR fragment (mutant) + agonist<br /> | + | **[[2y00]], [[2y02]], [[2y03]], [[2y04]], [[2vt4]], [[4ami]], [[4amj]] - tB1AR fragment (mutant) + agonist<br /> |
| - | [[2ycw]], [[2ycx]], [[2ycz]], [[2ycy]] - tB1AR fragment (mutant) + antagonist<br /> | + | **[[2ycw]], [[2ycx]], [[2ycz]], [[2ycy]] - tB1AR fragment (mutant) + antagonist<br /> |
| - | [[3zpq]], [[3zpr]] - tB1AR fragment (mutant) + aryl piperazine inhibitor<br /> | + | **[[3zpq]], [[3zpr]] - tB1AR fragment (mutant) + aryl piperazine inhibitor<br /> |
| - | + | *β-2 adrenergic receptor | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | **[[3pds]] - hB2AR/T4 lysozyme<br /> | ||
| + | **[[3p0g]] – hB2AR/T4 lysozyme + cameloid antibody fragment<br /> | ||
| + | **[[4lde]], [[4ldl]] - hB2AR/T4 lysozyme (mutant) + cameloid antibody fragment<br /> | ||
| + | **[[2r4s]],[[2r4r]] - hB2AR + Fab5 complex<br /> | ||
| + | **[[2rh1]] - hB2AR/T4 lysozyme (mutant)<br /> | ||
| + | **[[3d4s]] - hB2AR/T4 lysozyme (mutant) + cholesterol<br /> | ||
| + | **[[4gbr]] - hB2AR/T4 lysozyme (mutant) + carazolol<br /> | ||
| + | **[3ny8]], [[3ny9]], [[3nya]] – hB2AR/T4 lysozyme + agonist<br /> | ||
| + | **[[3sn6]] - human β-2 adrenergic receptor + cameloid antibody fragment bound by guanine nucleotide-binding protein G<br /> | ||
| + | **[[3kj6]], [[2r4r]], [[2r4s]] – hB2AR + FAB heavy+light chains<br /> | ||
| + | }} | ||
==References and Notes== | ==References and Notes== | ||
<references/> | <references/> | ||
Revision as of 12:01, 12 November 2014
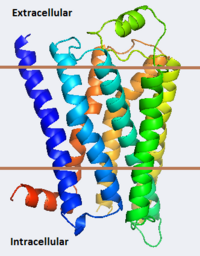
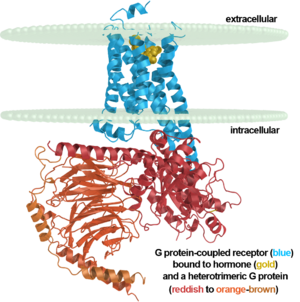
The adrenergic receptors are metabolic G protein-coupled receptors. They are the targets of catecholamines. The binding of an agonist to them causes a sympathetic response. The α-2 adrenergic receptor (A2AR) inhibits insulin or glucagons release. The β-1 adrenergic receptor (B1AR) increases cardiac output and secretion of rennin and ghrelin. The β-2 adrenergic receptor (B2AR) triggers many relaxation reactions. The images at the left and at the right correspond to one representative Adrenergic receptor, i.e. the crystal structure of human β-2 adrenergic Receptor (2rh1). See also
(morph was taken from Gallery of Morphs of the Yale Morph Server).
β2 adrenergic receptor binding a hormone analog |
| |||||||||||
| |||||||||||
This is the first structure of an activated GPCR in a complex with its G protein.
See also The Madison West High School 2008 SMART Team's Page on the β-2 adrenergic receptor
Contents |
Nobel Prize Related to the Structures
Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2r4r, 2r4s, & 2rh1 in 2007)[1][2][3] and the first activated GPCR in complex with its G protein (3sn6 in 2011)[4][5][6][7]. A detailed description of the laureates' body of work on this class of receptors with images is here.
3D Structures of Adrenergic receptor
Updated on 12-November-2014
References and Notes
See Also
- G protein-coupled receptor
- Beta-2 Adrenergic Receptor topic page
- Nobel Prizes for 3D Molecular Structure
- Highest impact structures of all time
- G proteins
- Rhodopsin
- GTP-binding protein
- Pharmaceutical Drugs
- Membrane proteins
- Hormone
External Resources
- Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2007) and the first activated GPCR in complex with its G protein (2011). A detailed description of the laureates' body of work on this class of receptors with images is here.
- The April 2008 RCSB PDB Molecule of the Month feature on Adrenergic Receptors by David S. Goodsell is 10.2210/rcsb_pdb/mom_2008_4.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Wayne Decatur, Karsten Theis, Alexander Berchansky