Sandbox Reserved 1086
From Proteopedia
| Line 12: | Line 12: | ||
== Exploring the Structure == | == Exploring the Structure == | ||
| + | |||
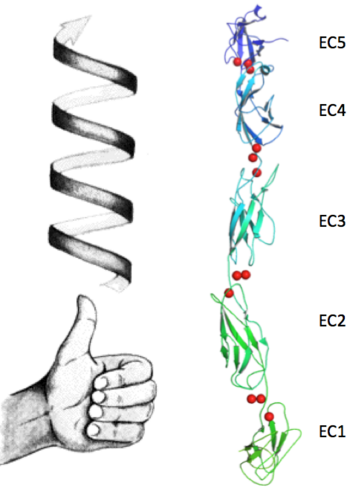
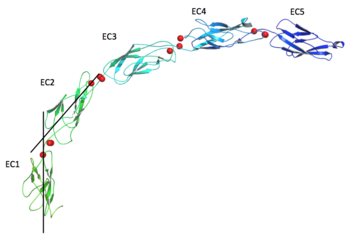
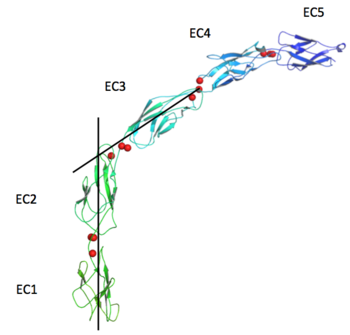
| + | The extracellular domain of C-cadherin is composed of five ectodomains repeated in tandem and linked by short peptide linkers. Each ectodomain (EC) shares the same ''greek key'' fold characteristic of immunoglobulin. The interfaces between the EC are calcium binding sites coordinating three ions each through the highly conserved motif Asp-X-Asp, Leu-Asp-Arg-Glu and Asp-X-Asn-Asp-Asn. The binding of calcium it though to confer rigidity and the classical curved shape to the structure and this is why it´s very suggested to be a triggering if not even compulsory step to the adhesion process. | ||
'''GFP''' is a beta barrel protein with 11 beta sheets. It is a 26.9kDa protein made up of 238 amino acids. The chromophore, responsible for the fluorescent properties of the protein, is buried inside the beta barrel as part of the central alpha helix passing through the barrel. The <scene name='69/699999/This_name_is_dumb/1'>chromophore</scene> forms via spontaneous cyclization and oxidation of three residues in the central alpha helix: -Thr65 (or Ser65)-Tyr66-Gly67. This cyclization and oxidation creates the chromophore's five-membered ring via a new bond between the threonine and the glycine residues <ref>PMID:8703075</ref>. | '''GFP''' is a beta barrel protein with 11 beta sheets. It is a 26.9kDa protein made up of 238 amino acids. The chromophore, responsible for the fluorescent properties of the protein, is buried inside the beta barrel as part of the central alpha helix passing through the barrel. The <scene name='69/699999/This_name_is_dumb/1'>chromophore</scene> forms via spontaneous cyclization and oxidation of three residues in the central alpha helix: -Thr65 (or Ser65)-Tyr66-Gly67. This cyclization and oxidation creates the chromophore's five-membered ring via a new bond between the threonine and the glycine residues <ref>PMID:8703075</ref>. | ||
Revision as of 07:25, 21 April 2015
| This Sandbox is Reserved from 15/04/2015, through 15/06/2015 for use in the course "Protein structure, function and folding" taught by Taru Meri at the University of Helsinki. This reservation includes Sandbox Reserved 1081 through Sandbox Reserved 1090. |
To get started:
More help: Help:Editing |
Contents |
C-cadherin
| |||||||||||
Exploring the Structure
The extracellular domain of C-cadherin is composed of five ectodomains repeated in tandem and linked by short peptide linkers. Each ectodomain (EC) shares the same greek key fold characteristic of immunoglobulin. The interfaces between the EC are calcium binding sites coordinating three ions each through the highly conserved motif Asp-X-Asp, Leu-Asp-Arg-Glu and Asp-X-Asn-Asp-Asn. The binding of calcium it though to confer rigidity and the classical curved shape to the structure and this is why it´s very suggested to be a triggering if not even compulsory step to the adhesion process.
GFP is a beta barrel protein with 11 beta sheets. It is a 26.9kDa protein made up of 238 amino acids. The chromophore, responsible for the fluorescent properties of the protein, is buried inside the beta barrel as part of the central alpha helix passing through the barrel. The forms via spontaneous cyclization and oxidation of three residues in the central alpha helix: -Thr65 (or Ser65)-Tyr66-Gly67. This cyclization and oxidation creates the chromophore's five-membered ring via a new bond between the threonine and the glycine residues [1].
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
.png|thumb|right|350px|Abspregram of the 151 species]]This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. [[Image: This is a sample scene created with SAT to by Group, and another to make of the protein.
Structural highlights
| 1q5a is a 2 chain structure with sequence from Mus musculus. The March 2008 RCSB PDB Molecule of the Month feature on Cadherin by David S. Goodsell is 10.2210/rcsb_pdb/mom_2008_3. Full crystallographic information is available from OCA. For a guided tour on the structure components use FirstGlance. | |
| Ligands: | , , |
| Related: | 1l3w, 1q55, 1q5b, 1q5c |
| Resources: | FirstGlance, OCA, RCSB, PDBsum |