We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1083
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Assembly within biological system == | == Assembly within biological system == | ||
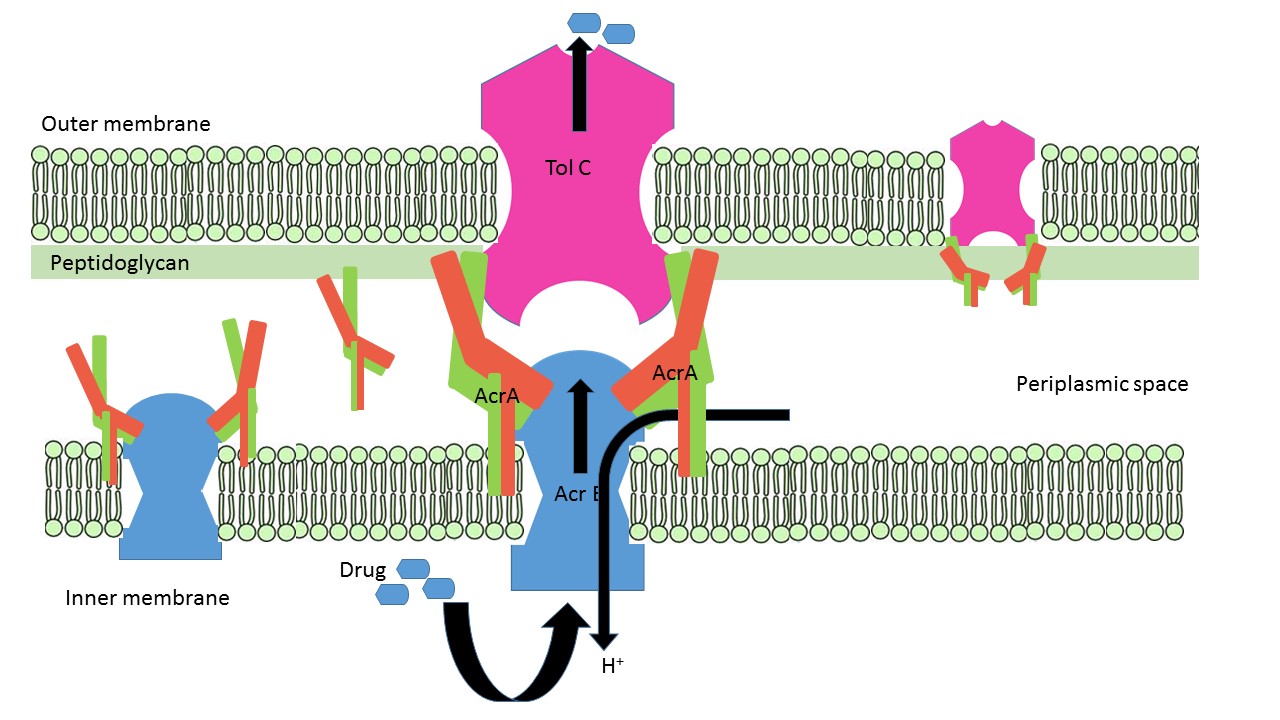
| - | AcrA is present within E. coli cells as a part of tripartite membrane associated efflux system along with [http://www.uniprot.org/uniprot/P31224 AcrB] and [http://www.uniprot.org/uniprot/P02930 TolC]. AcrA is present in the periplasmic space of cell with the proton antiporter AcrB in the inner-membrane and channel TolC in the outer membrane. It can it can remain free or form bipartite complexes with AcrB and TolC. The lipoyl and β-barrel domain of AcrA interact with AcrB, whereas the α-helical hairpin domain interact with TolC | + | AcrA is present within E. coli cells as a part of tripartite membrane associated efflux system along with [http://www.uniprot.org/uniprot/P31224 AcrB] and [http://www.uniprot.org/uniprot/P02930 TolC]. AcrA is present in the periplasmic space of cell with the proton antiporter AcrB in the inner-membrane and channel TolC in the outer membrane. It can it can remain free or form bipartite complexes with AcrB and TolC. The lipoyl and β-barrel domain of AcrA interact with AcrB, whereas the α-helical hairpin domain interact with TolC <ref>PMID:19411330</ref>. [[Image: AcrA_AcrB_TolC_assembly.jpg]] |
ArcA remains attached to the inner membrane via lipid acylation of Cys-25. N and C termini of AcrA form two <scene name='69/699996/Membrane_proximal/1'>apposing β-stands</scene>, β1 (54-61) and β14 (292-297)lies proximal to inner membrane. A 28 flexible residues connects acylated Cys-25 with the β-barrel domain and allow the protein to reach the periplasmic top of AcrB (Yu et al 2003). A <scene name='69/699996/Short_alpha_helix/2'>short α-helix</scene> (222-230) located between β-10 (in red) and β-11 (in blue) closes off the end of the β-barrel near the C-terminus of AcrA fragment. Approximately 100 residues in the C-terminal are predicted to be important for AcrAB-TolC interaction. | ArcA remains attached to the inner membrane via lipid acylation of Cys-25. N and C termini of AcrA form two <scene name='69/699996/Membrane_proximal/1'>apposing β-stands</scene>, β1 (54-61) and β14 (292-297)lies proximal to inner membrane. A 28 flexible residues connects acylated Cys-25 with the β-barrel domain and allow the protein to reach the periplasmic top of AcrB (Yu et al 2003). A <scene name='69/699996/Short_alpha_helix/2'>short α-helix</scene> (222-230) located between β-10 (in red) and β-11 (in blue) closes off the end of the β-barrel near the C-terminus of AcrA fragment. Approximately 100 residues in the C-terminal are predicted to be important for AcrAB-TolC interaction. | ||
Revision as of 06:56, 23 April 2015
| This Sandbox is Reserved from 15/04/2015, through 15/06/2015 for use in the course "Protein structure, function and folding" taught by Taru Meri at the University of Helsinki. This reservation includes Sandbox Reserved 1081 through Sandbox Reserved 1090. |
To get started:
More help: Help:Editing |
AcrA
| |||||||||||
References
- ↑ Putman M, van Veen HW, Konings WN. Molecular properties of bacterial multidrug transporters. Microbiol Mol Biol Rev. 2000 Dec;64(4):672-93. PMID:11104814
- ↑ Kumar A, Schweizer HP. Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv Drug Deliv Rev. 2005 Jul 29;57(10):1486-513. PMID:15939505 doi:http://dx.doi.org/10.1016/j.addr.2005.04.004
- ↑ Mikolosko J, Bobyk K, Zgurskaya HI, Ghosh P. Conformational flexibility in the multidrug efflux system protein AcrA. Structure. 2006 Mar;14(3):577-87. PMID:16531241 doi:10.1016/j.str.2005.11.015
- ↑ Johnson JM, Church GM. Alignment and structure prediction of divergent protein families: periplasmic and outer membrane proteins of bacterial efflux pumps. J Mol Biol. 1999 Apr 2;287(3):695-715. PMID:10092468 doi:http://dx.doi.org/10.1006/jmbi.1999.2630
- ↑ Akama H, Kanemaki M, Yoshimura M, Tsukihara T, Kashiwagi T, Yoneyama H, Narita S, Nakagawa A, Nakae T. Crystal structure of the drug discharge outer membrane protein, OprM, of Pseudomonas aeruginosa: dual modes of membrane anchoring and occluded cavity end. J Biol Chem. 2004 Dec 17;279(51):52816-9. Epub 2004 Oct 26. PMID:15507433 doi:10.1074/jbc.C400445200
- ↑ Ge Q, Yamada Y, Zgurskaya H. The C-terminal domain of AcrA is essential for the assembly and function of the multidrug efflux pump AcrAB-TolC. J Bacteriol. 2009 Jul;191(13):4365-71. doi: 10.1128/JB.00204-09. Epub 2009 May 1. PMID:19411330 doi:http://dx.doi.org/10.1128/JB.00204-09