We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1085
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
[[Image: Cleavage site of RNase III.png|thumb|right|320px|Fig.3. ''cleavage site of Aa-RNase III'' ]] | [[Image: Cleavage site of RNase III.png|thumb|right|320px|Fig.3. ''cleavage site of Aa-RNase III'' ]] | ||
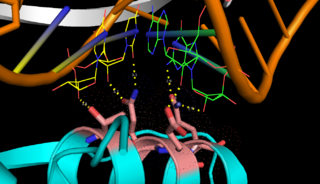
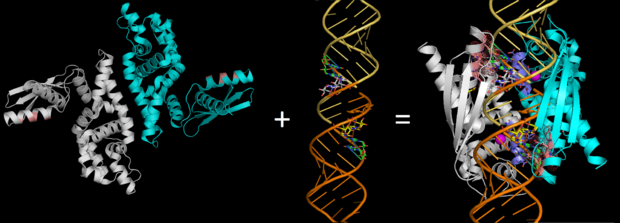
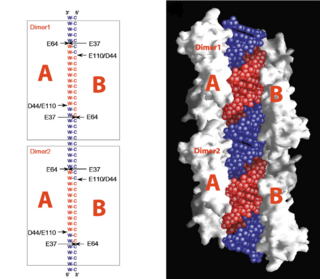
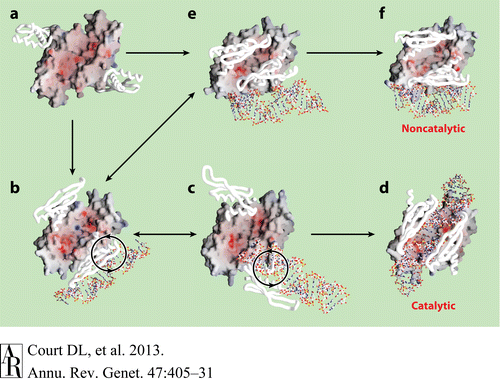
| - | On the basis of the structural and biochemical data, catalytic models were proposed before the structure of a catalytic complex became available. Comparing the structure of Aa-RNase III with the structure of RNA-free Thermotoga maritima RNase III (RNA-free Tm-RNase III, PDB ID code 1O0W)<ref name= Gan>Gan, J., et al., Intermediate states of ribonuclease III in complex with double-stranded RNA. Structure, 2005. 13(10): p. 1435-1442.</ref> shows that there is <scene name='69/699998/Linker_rotation/1'>dramatic rotation</scene> and shift of dsRBD due to RNA binding, and there is a <scene name='69/699998/Linker/2'>flexible linker</scene> KGEMLFD between endoRD and dsRBD leading the rotation happened (''Fig. | + | On the basis of the structural and biochemical data, catalytic models were proposed before the structure of a catalytic complex became available. Comparing the structure of Aa-RNase III with the structure of RNA-free Thermotoga maritima RNase III (RNA-free Tm-RNase III, PDB ID code 1O0W)<ref name= Gan>Gan, J., et al., Intermediate states of ribonuclease III in complex with double-stranded RNA. Structure, 2005. 13(10): p. 1435-1442.</ref> shows that there is <scene name='69/699998/Linker_rotation/1'>dramatic rotation</scene> and shift of dsRBD due to RNA binding, and there is a <scene name='69/699998/Linker/2'>flexible linker</scene> KGEMLFD between endoRD and dsRBD leading the rotation happened (''Fig. 2''). In addition, the two dsRBD domains are spaced apart from each other in a way that allows free rotation of dsRBD around the linker. In vivo data suggested that E110, E37, D44, and E64 are essential for catalysis<ref>Blaszczyk, J., et al., Noncatalytic assembly of ribonuclease III with double-stranded RNA. Structure, 2004. 12(3): p. 457-466.</ref>. This led to the model of the proteins active centers, which can accommodate a dsRNA substrate, each containing two different RNA cleavage sites, <scene name='69/699998/D44_e110_e37_e64/1'>D44/E110 and E37/E64</scene> (pink and red). Specifically, E64 from each partner subunit, along with E37, E110, and D44 are located in the signature motif located at each end of the catalytic valley<ref>Blaszczyk, J., et al., Crystallographic and modeling studies of RNase III suggest a mechanism for double-stranded RNA cleavage. Structure, 2001. 9(12): p. 1225-1236</ref>. ''Fig. 3'' presents a model showing Aa-RNase III cleaving dsRNA. This produces two identical RNA strands, each containing a 2 bp 3' overhang. |
Revision as of 05:32, 24 April 2015
| This Sandbox is Reserved from 15/04/2015, through 15/06/2015 for use in the course "Protein structure, function and folding" taught by Taru Meri at the University of Helsinki. This reservation includes Sandbox Reserved 1081 through Sandbox Reserved 1090. |
To get started:
More help: Help:Editing |
Endoribonuclease III
| |||||||||||
References
- ↑ Lioliou E, Sharma CM, Caldelari I, et al. Global Regulatory Functions of the Staphylococcus aureus Endoribonuclease III in Gene Expression. Hughes D, ed. PLoS Genetics. 2012;8(6):e1002782. doi:10.1371/journal.pgen.1002782
- ↑ Lamontagne, B., et al., The RNase III family: a conserved structure and expanding functions in eukaryotic dsRNA metabolism. Yeast, 2001. 45(191): p. 154-158.
- ↑ Robertson, H.D., Escherichia coli ribonuclease III cleavage sites. Cell, 1982. 30(3): p. 669-672.
- ↑ Grunberg-Manago, M., Messenger RNA stability and its role in control of gene expression in bacteria and phages. Annual review of genetics, 1999. 33(1): p. 193-227.
- ↑ 5.0 5.1 Gan, J., et al., Intermediate states of ribonuclease III in complex with double-stranded RNA. Structure, 2005. 13(10): p. 1435-1442.
- ↑ Blaszczyk, J., et al., Noncatalytic assembly of ribonuclease III with double-stranded RNA. Structure, 2004. 12(3): p. 457-466.
- ↑ Blaszczyk, J., et al., Crystallographic and modeling studies of RNase III suggest a mechanism for double-stranded RNA cleavage. Structure, 2001. 9(12): p. 1225-1236
- ↑ Blaszczyk, J., et al., Noncatalytic assembly of ribonuclease III with double-stranded RNA. Structure, 2004. 12(3): p. 457-466
- ↑ Gan, J., et al., Structural insight into the mechanism of double-stranded RNA processing by ribonuclease III. Cell, 2006. 124(2): p. 355-66