We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1084
From Proteopedia
(Difference between revisions)
| Line 39: | Line 39: | ||
Aside from the catalytic Zn2+ ion, the enzyme activity of HDAC8 is dependent on the presence of the monovalent ion, K+/Na+ [60]. The crystal structure of HDAC8 shows the presence of two binding sites for K+/Na+ [58]. The <scene name='69/699997/K1_site/4'>first K+/Na+ binding site (K1)</scene> is located in the vicinity of the enzyme catalytic machinery, and it is hexacoordinated (octahedral geometry) with His 180 (carbonyl oxygen of the main chain), Asp 178 (oxygen atom of the main chain and side chain), Leu 200 (carbonyl oxygen of the main chain), and Ser 199 (O). Notably, His 180 and Asp 178 are the common residues coordinated with both the catalytic Zn2+ as well as the K+/Na+ ion. The <scene name='69/699997/K2_site/4'>second binding site for K+/Na+ ion (K2)</scene> is located near the surface. It is hexacoordinated (octahedral geometry) with F189, T192, V195, Y225 as well as two water molecule. | Aside from the catalytic Zn2+ ion, the enzyme activity of HDAC8 is dependent on the presence of the monovalent ion, K+/Na+ [60]. The crystal structure of HDAC8 shows the presence of two binding sites for K+/Na+ [58]. The <scene name='69/699997/K1_site/4'>first K+/Na+ binding site (K1)</scene> is located in the vicinity of the enzyme catalytic machinery, and it is hexacoordinated (octahedral geometry) with His 180 (carbonyl oxygen of the main chain), Asp 178 (oxygen atom of the main chain and side chain), Leu 200 (carbonyl oxygen of the main chain), and Ser 199 (O). Notably, His 180 and Asp 178 are the common residues coordinated with both the catalytic Zn2+ as well as the K+/Na+ ion. The <scene name='69/699997/K2_site/4'>second binding site for K+/Na+ ion (K2)</scene> is located near the surface. It is hexacoordinated (octahedral geometry) with F189, T192, V195, Y225 as well as two water molecule. | ||
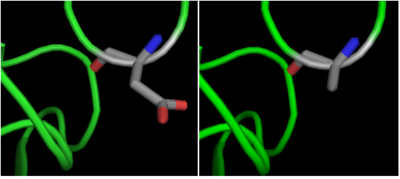
| - | The crystal structure of <scene name='69/699997/Hdac8_binding_substrate/3'>HDAC8-substrate complex</scene> shows that aspartate residue <scene name='69/699997/Hdac8_d101/2'>(D101)</scene> is important in the substrate binding <ref>Vannini, A. et al. Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports 8, 879–884 (2007).</ref>. The Asp 101 carboxylate moiety makes two consecutive hydrogen bonds with the backbone of the deacetylated peptide substrate. Mutation of Asp 101 to Ala inhibits HDAC8 activity. The Asp residue has been found to be strictly conserved among different HDAC isozymes.[[Image:D101.png]] | + | The crystal structure of <scene name='69/699997/Hdac8_binding_substrate/3'>HDAC8-substrate complex</scene> shows that aspartate residue <scene name='69/699997/Hdac8_d101/2'>(D101)</scene> is important in the substrate binding <ref>Vannini, A. et al. Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports 8, 879–884 (2007).</ref>. The Asp 101 carboxylate moiety makes two consecutive hydrogen bonds with the backbone of the deacetylated peptide substrate. Mutation of Asp 101 to Ala inhibits HDAC8 activity. The Asp residue has been found to be strictly conserved among different HDAC isozymes. |
| + | [[Image:D101.png|400px|left|thumb| '''Fig. 2''' Mutation of Asp 101 to Ala inhibits HDAC8 activity.]] | ||
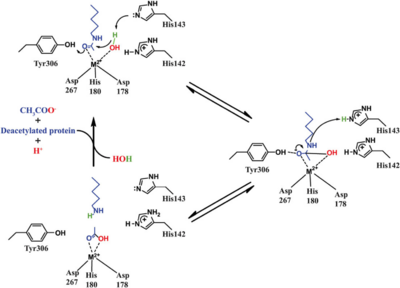
Based on the crystallographic studies, a '''mechanism''' of the HDAC8 catalyzed reaction has been proposed. | Based on the crystallographic studies, a '''mechanism''' of the HDAC8 catalyzed reaction has been proposed. | ||
Revision as of 17:31, 23 April 2015
| This Sandbox is Reserved from 15/04/2015, through 15/06/2015 for use in the course "Protein structure, function and folding" taught by Taru Meri at the University of Helsinki. This reservation includes Sandbox Reserved 1081 through Sandbox Reserved 1090. |
To get started:
More help: Help:Editing |
Histone Deacetylase 8
| |||||||||||
References
- ↑ Ramakrishnan, V. Histone Structure and the Organization of the Nucleosome. Annual Review of Biophysics and Biomolecular Structure 26, 83–112 (1997).
- ↑ Waltregny, D. et al. Histone deacetylase HDAC8 associates with smooth muscle alpha-actin and is essential for smooth muscle cell contractility. FASEB J. 19, 966–968 (2005).
- ↑ Haberland, M., Mokalled, M. H., Montgomery, R. L. & Olson, E. N. Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes Dev. 23, 1625–1630 (2009).
- ↑ Gräff, J. & Tsai, L.-H. The Potential of HDAC Inhibitors as Cognitive Enhancers. Annual Review of Pharmacology and Toxicology 53, 311–330 (2013).
- ↑ Blagosklonny, M. V. et al. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol. Cancer Ther. 1, 937–941 (2002).
- ↑ 6.0 6.1 6.2 Yan, W. et al. Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene 32, 599–609 (2013).
- ↑ Dokmanovic, M., Clarke, C. & Marks, P. A. Histone Deacetylase Inhibitors: Overview and Perspectives. Mol Cancer Res 5, 981–989 (2007).
- ↑ Bolden, J. E., Peart, M. J. & Johnstone, R. W. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5, 769–784 (2006).
- ↑ 9.0 9.1 Deardorff, M. A. et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 489, 313–317 (2012).
- ↑ Ito, K. et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 352, 1967–1976 (2005).
- ↑ Vannini, A. et al. Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports 8, 879–884 (2007).
- ↑ 12.0 12.1 Somoza, J. R. et al. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure 12, 1325–1334 (2004).