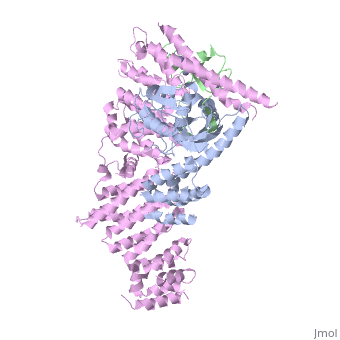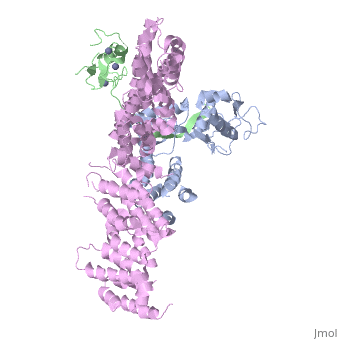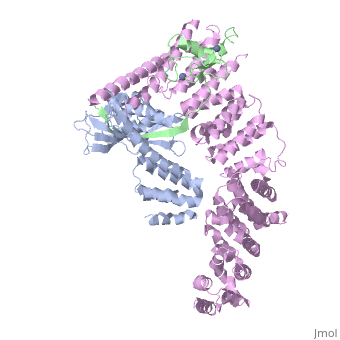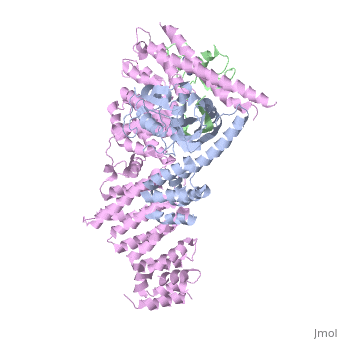We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
RING box protein
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='4f52' size='340' side='right' caption='Human RING finger protein 1 (green | + | <StructureSection load='4f52' size='340' side='right' caption='Human RING finger protein 1 (green) complex with cullin-1 (grey), glomulin (magenta) and Zn+2 ions (grey) (PDB code [[4f52]])' scene=''> |
'''RING box protein''' (RBX) makes a heterodimer with cullin. The heterodimer is essential for the protein ubiquitination process. RBX1 is a component of the SCF (Skp1-cullin-F box protein) E3 ubiquitin ligase complex. RBX contains a RING-type zinc finger domain. The RING domain is 40 to 60 residues long and binds two Zn atoms. It is involved in protein-protein interaction. | '''RING box protein''' (RBX) makes a heterodimer with cullin. The heterodimer is essential for the protein ubiquitination process. RBX1 is a component of the SCF (Skp1-cullin-F box protein) E3 ubiquitin ligase complex. RBX contains a RING-type zinc finger domain. The RING domain is 40 to 60 residues long and binds two Zn atoms. It is involved in protein-protein interaction. | ||
Revision as of 09:10, 17 August 2016
| |||||||||||
3D Structures of RING box protein
Updated on 17-August-2016