Tobacco Etch Virus (TEV) Protease
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
===Enzymatic Mechanism=== | ===Enzymatic Mechanism=== | ||
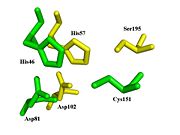
| - | + | [[Image:Triad.jpg|left|thumb|Spatial comparison of the catalytic triads from bovine <span style="color:yellow;background-color:black;font-weight:bold;">chymotrypsin</span> and the <span style="color:green;background-color:black;font-weight:bold;">TEV protease</span>. PDB Files: [http://proteopedia.org/wiki/index.php/1yph 1YPH] and [http://proteopedia.org/wiki/index.php/1lvm 1LVM].]] | |
| + | {{Clear}} | ||
| + | Given the catalytic cysteine residue yet the overall serine protease-like fold possessed by 3C-type cysteine proteases like the TEV protease, it has long been debated whether these proteases (from both the Picornaviruses and Potyviruses) catalyze the cleavage of a peptide bond in a manner similar to conventional cysteine proteases (e.g., the plant enzyme papain) or via a similar mechanism as serine proteases (e.g., chymotrypsin). Traditional cysteine proteases utilize a thiolate-imidizolium ion pair to function which ultimately gives rise to the characteristic bell-shaped pH dependency observed of such proteases. On the other hand, traditional serine proteases utilize a characteristic Ser-His-Asp catalytic triad. The two types of proteases display a number of well-described mechanistic differences. | ||
Due to the similar spatial arrangement of catalytic residues, proteolysis by the TEV protease (and other 3C-type viral proteases) is thought to occur through general base assisted nucleophilic catalysis homologous to chymotrypsin-like serine proteases and analogous to papain-like cysteine proteases <ref name="Yin" >PMID:17599357</ref> <ref>PMID:8320292</ref>. However, the exact details of the catalytic mechanism of the TEV protease have yet to be elucidated. Provided is a brief description of the proposed catalytic mechanism-closely resembling that of chymotrypsin-like serine proteases. | Due to the similar spatial arrangement of catalytic residues, proteolysis by the TEV protease (and other 3C-type viral proteases) is thought to occur through general base assisted nucleophilic catalysis homologous to chymotrypsin-like serine proteases and analogous to papain-like cysteine proteases <ref name="Yin" >PMID:17599357</ref> <ref>PMID:8320292</ref>. However, the exact details of the catalytic mechanism of the TEV protease have yet to be elucidated. Provided is a brief description of the proposed catalytic mechanism-closely resembling that of chymotrypsin-like serine proteases. | ||
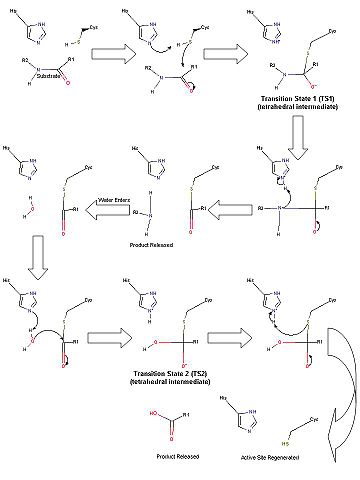
| - | [[Image:TEVMechanism.jpg|thumb|upright=2 |right |Scheme 1. Proposed catalytic mechanism of the TEV protease.]] | + | [[Image:TEVMechanism.jpg|thumb|upright=2 |right |Scheme 1. Proposed catalytic mechanism of the TEV protease.]] |
| + | {{Clear}} | ||
In the first half of the reaction (please refer to Scheme 1), the acylation step, the catalytic histidine (His46 in TEV protease numbering) acts as a general base to abstract a proton from Cys151 and produce the catalytic thiolate. (This is a major difference between chymotrypsin-like cysteine proteases and traditional cysteine proteases; the role of the catalytic His in traditional cysteine proteases is not to abstract a proton from the catalytic cysteine residue, but instead to donate a proton to the substrate.) This thiolate nucleophile then attacks the carbonyl atom of the scissile bond, ultimately leading to the formation of the first tetrahedral intermediate (TS1). The negative oxygen ion, after accepting electrons from the carbonyl double bond, is stabilized through a network of hydrogen bond interactions with the backbone atoms of residues 149 and 151 in what is termed the [http://en.wikipedia.org/wiki/Serine_protease oxyanion hole]. This stabilization of the negative charge generated in the TS is a critical part in the catalytic mechanism. In fact, most viral 3C-type cysteine proteases contain highly conserved small amino acids around the catalytic cysteine residue in order to properly orient the residue for nucleophilic attack as well as properly position the backbone atoms for the formation of the oxyanion hole. Collapse of this intermediate leads to release of the C-terminal product fragment and a covalent thioester enzyme-substrate complex. The second half of the reaction involves subsequent release of the N-terminal product fragment and regeneration of the enzyme in what is called the deacylation step. To accomplish this, a water molecule (activated by His46 to a nucleophilic OH-) attacks the thioester carbonyl carbon, forming the second tetrahedral intermediate (T2). As with T1, this second transition state is stabilized by the oxyanion hole within the enzyme. Breakdown of the second transition state regenerates the catalytic cysteine residue and cleavage of the target peptide bond is complete <ref name="Yin"/>. Importantly, the aspartic acid residue of the catalytic triad (Asp81) serves to correctly orient the histidine residue (His46) throughout the catalytic process. | In the first half of the reaction (please refer to Scheme 1), the acylation step, the catalytic histidine (His46 in TEV protease numbering) acts as a general base to abstract a proton from Cys151 and produce the catalytic thiolate. (This is a major difference between chymotrypsin-like cysteine proteases and traditional cysteine proteases; the role of the catalytic His in traditional cysteine proteases is not to abstract a proton from the catalytic cysteine residue, but instead to donate a proton to the substrate.) This thiolate nucleophile then attacks the carbonyl atom of the scissile bond, ultimately leading to the formation of the first tetrahedral intermediate (TS1). The negative oxygen ion, after accepting electrons from the carbonyl double bond, is stabilized through a network of hydrogen bond interactions with the backbone atoms of residues 149 and 151 in what is termed the [http://en.wikipedia.org/wiki/Serine_protease oxyanion hole]. This stabilization of the negative charge generated in the TS is a critical part in the catalytic mechanism. In fact, most viral 3C-type cysteine proteases contain highly conserved small amino acids around the catalytic cysteine residue in order to properly orient the residue for nucleophilic attack as well as properly position the backbone atoms for the formation of the oxyanion hole. Collapse of this intermediate leads to release of the C-terminal product fragment and a covalent thioester enzyme-substrate complex. The second half of the reaction involves subsequent release of the N-terminal product fragment and regeneration of the enzyme in what is called the deacylation step. To accomplish this, a water molecule (activated by His46 to a nucleophilic OH-) attacks the thioester carbonyl carbon, forming the second tetrahedral intermediate (T2). As with T1, this second transition state is stabilized by the oxyanion hole within the enzyme. Breakdown of the second transition state regenerates the catalytic cysteine residue and cleavage of the target peptide bond is complete <ref name="Yin"/>. Importantly, the aspartic acid residue of the catalytic triad (Asp81) serves to correctly orient the histidine residue (His46) throughout the catalytic process. | ||
====Low-Barrier Hydrogen Bond==== | ====Low-Barrier Hydrogen Bond==== | ||
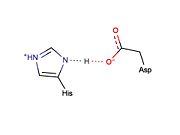
[[Image:LBHB2.jpg|thumb|left|Potential LBHB formed between catalytic residues His46 and Asp81 that may aid in catalysis through stabilization of the tetrahedral intermediate (based on mechanistic homology with chymotrypsin).]] | [[Image:LBHB2.jpg|thumb|left|Potential LBHB formed between catalytic residues His46 and Asp81 that may aid in catalysis through stabilization of the tetrahedral intermediate (based on mechanistic homology with chymotrypsin).]] | ||
| + | {{Clear}} | ||
[http://en.wikipedia.org/wiki/Low-barrier_hydrogen_bond Low-barrier hydrogen bonds] (LBHBs) are short, strong hydrogen bonds thought to be formed under certain circumstances and play a role in enzymatic catalysis <ref>PMID:9748211</ref>. Although the existence of LBHBs is highly debated, a great deal of spectroscopic evidence suggests that a LBHB may form between the catalytic His and Asp residues during the mechanism of chymotrypsin <ref name="Frey" >PMID:9109667</ref>. It is proposed that bridging of the proton between the His and Asp residue through a LBHB may aid in stabilization of the tetrahedral intermediate <ref name="Frey"/>. It is thought that the LBHB shared between the His and Asp residues of the catalytic triad in serine proteases increases the nucleophilicity of the catalytic serine essentially by increasing the basicity of the His residue. Traditional cysteine proteases (e.g., papain) are thought not to require the formation of a LBHB because: (1) they do not contain a counterpart to the catalytic Asp residue found in serine proteases and (2) the active site cysteine exists as a thiolate ion, which is highly nucleophilic and does not require its nucleophilicity to be enhanced to facilitate catalysis. Given that catalysis by the TEV protease is thought to occur in a manner similar to chymotrypsin, it is possible that a similar LBHB may form between the catalytic residues, His46 and Asp81, although there is no experimental spectroscopic evidence to support this hypothesis. However, X-ray crystal structures of the TEV protease may support LBHB formation between His46 and Asp181. Depending on the crystal structure, the distance between these two residues appears to vary between ~3.0 Å (typical of weak regular hydrogen bonds) and ~2.6 Å (typical of stronger LBHB). | [http://en.wikipedia.org/wiki/Low-barrier_hydrogen_bond Low-barrier hydrogen bonds] (LBHBs) are short, strong hydrogen bonds thought to be formed under certain circumstances and play a role in enzymatic catalysis <ref>PMID:9748211</ref>. Although the existence of LBHBs is highly debated, a great deal of spectroscopic evidence suggests that a LBHB may form between the catalytic His and Asp residues during the mechanism of chymotrypsin <ref name="Frey" >PMID:9109667</ref>. It is proposed that bridging of the proton between the His and Asp residue through a LBHB may aid in stabilization of the tetrahedral intermediate <ref name="Frey"/>. It is thought that the LBHB shared between the His and Asp residues of the catalytic triad in serine proteases increases the nucleophilicity of the catalytic serine essentially by increasing the basicity of the His residue. Traditional cysteine proteases (e.g., papain) are thought not to require the formation of a LBHB because: (1) they do not contain a counterpart to the catalytic Asp residue found in serine proteases and (2) the active site cysteine exists as a thiolate ion, which is highly nucleophilic and does not require its nucleophilicity to be enhanced to facilitate catalysis. Given that catalysis by the TEV protease is thought to occur in a manner similar to chymotrypsin, it is possible that a similar LBHB may form between the catalytic residues, His46 and Asp81, although there is no experimental spectroscopic evidence to support this hypothesis. However, X-ray crystal structures of the TEV protease may support LBHB formation between His46 and Asp181. Depending on the crystal structure, the distance between these two residues appears to vary between ~3.0 Å (typical of weak regular hydrogen bonds) and ~2.6 Å (typical of stronger LBHB). | ||
===Substrate Specificity=== | ===Substrate Specificity=== | ||
| - | + | [[Image:Substrate.jpg|right|thumb|TEV protease substrate recognition sequence and numbering.]] | |
| + | {{Clear}} | ||
| + | The canonical recognition site of the TEV protease is the seven amino acid sequence ENLYFQ/G, with cleavage occurring after the glutamine residue. All 3C type proteases exhibit a strong preference for glutamine at the P1 position and for small aliphatic residues (glycine or serine) at the P1’ position <ref name="Phan" />. However, the requirement for a Gly or Ser in the P1’ position may not be as stringent as originally thought. Kapust et al. substituted this position with various amino acids and assessed the impact the P1’ position had on the catalytic efficiency of the TEV protease <ref name="Kapust2">PMID:12074568</ref>. The amino acids tested were ranked as follows: | ||
:::G>S>A>M>C>N>H>Y>K>D>Q>F>T>W>R>L>E>I>V>P | :::G>S>A>M>C>N>H>Y>K>D>Q>F>T>W>R>L>E>I>V>P | ||
Revision as of 10:44, 9 September 2015
| |||||||||||
3D structures of TEV protease
1q31 – TEV (mutant) – Tobacco etch virus
1lvb, 1lvm – TEV catalytic domain (mutant) + oligopeptide substrate
Additional Resources
For additional information, See: Viral Infections
References
- ↑ Ryan MD, Flint M. Virus-encoded proteinases of the picornavirus super-group. J Gen Virol. 1997 Apr;78 ( Pt 4):699-723. PMID:9129643
- ↑ Stanway G. Structure, function and evolution of picornaviruses. J Gen Virol. 1990 Nov;71 ( Pt 11):2483-501. PMID:2254747
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Phan J, Zdanov A, Evdokimov AG, Tropea JE, Peters HK 3rd, Kapust RB, Li M, Wlodawer A, Waugh DS. Structural basis for the substrate specificity of tobacco etch virus protease. J Biol Chem. 2002 Dec 27;277(52):50564-72. Epub 2002 Oct 10. PMID:12377789 doi:http://dx.doi.org/10.1074/jbc.M207224200
- ↑ 4.0 4.1 Yin J, Niu C, Cherney MM, Zhang J, Huitema C, Eltis LD, Vederas JC, James MN. A mechanistic view of enzyme inhibition and peptide hydrolysis in the active site of the SARS-CoV 3C-like peptidase. J Mol Biol. 2007 Aug 24;371(4):1060-74. Epub 2007 Jun 8. PMID:17599357 doi:10.1016/j.jmb.2007.06.001
- ↑ Arad D, Kreisberg R, Shokhen M. Structural and mechanistic aspects of 3C proteases from the Picornavirus family. J Chem Inf Comput Sci. 1993 May-Jun;33(3):345-9. PMID:8320292
- ↑ Cleland WW, Frey PA, Gerlt JA. The low barrier hydrogen bond in enzymatic catalysis. J Biol Chem. 1998 Oct 2;273(40):25529-32. PMID:9748211
- ↑ 7.0 7.1 Cassidy CS, Lin J, Frey PA. A new concept for the mechanism of action of chymotrypsin: the role of the low-barrier hydrogen bond. Biochemistry. 1997 Apr 15;36(15):4576-84. PMID:9109667 doi:10.1021/bi962013o
- ↑ 8.0 8.1 8.2 Kapust RB, Tozser J, Copeland TD, Waugh DS. The P1' specificity of tobacco etch virus protease. Biochem Biophys Res Commun. 2002 Jun 28;294(5):949-55. PMID:12074568 doi:10.1016/S0006-291X(02)00574-0
- ↑ 9.0 9.1 Nunn CM, Jeeves M, Cliff MJ, Urquhart GT, George RR, Chao LH, Tscuchia Y, Djordjevic S. Crystal structure of tobacco etch virus protease shows the protein C terminus bound within the active site. J Mol Biol. 2005 Jul 1;350(1):145-55. PMID:15919091 doi:10.1016/j.jmb.2005.04.013
- ↑ Mohanty AK, Simmons CR, Wiener MC. Inhibition of tobacco etch virus protease activity by detergents. Protein Expr Purif. 2003 Jan;27(1):109-14. PMID:12509992
- ↑ Kapust RB, Tozser J, Fox JD, Anderson DE, Cherry S, Copeland TD, Waugh DS. Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng. 2001 Dec;14(12):993-1000. PMID:11809930
- ↑ Gorbalenya AE, Blinov VM, Donchenko AP. Poliovirus-encoded proteinase 3C: a possible evolutionary link between cellular serine and cysteine proteinase families. FEBS Lett. 1986 Jan 6;194(2):253-7. PMID:3000829
Proteopedia Page Contributors and Editors (what is this?)
Ashley Steere, Alexander Berchansky, Michal Harel, Wayne Decatur, David Canner