User:Wade Cook/Sandbox 1
From Proteopedia
| Line 12: | Line 12: | ||
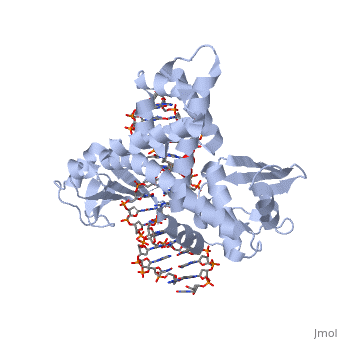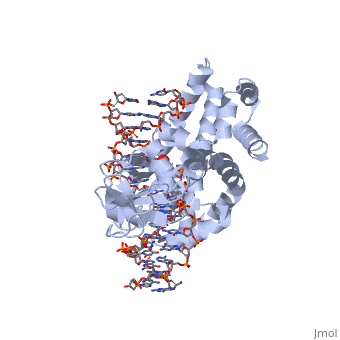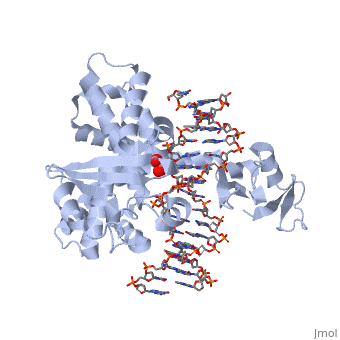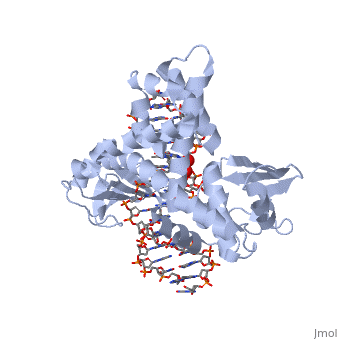
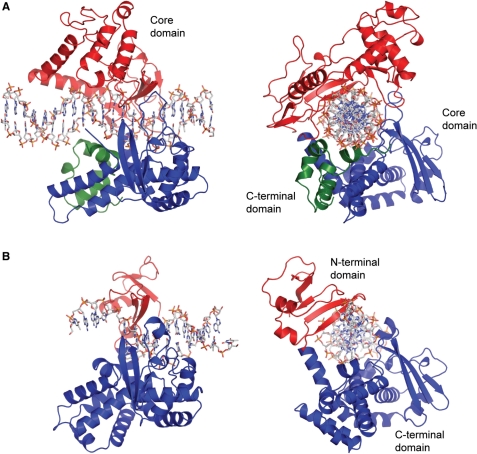
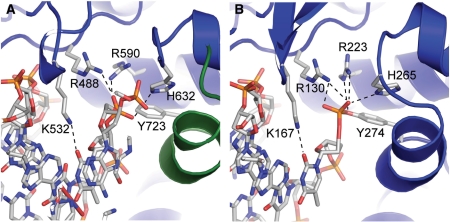
In the above figure, [A] shows the C-terminal and N-terminal domains of eukaryotic topoisomerase 1B. [B] shows the C-terminal and N-terminal domains of viral topoisomerase 1B. Both domains are highly conserved between the different species, however, the N-terminal domain of viral topoisomerase 1B is slightly smaller and more simple than the larger eukaryotic N-terminal domain. The type 1B topoisomerase can relieve negative or positive supercoiling without the use of ATP. As long as there's torsional strain on the DNA strand from the supercoiling, this is enough potential energy to drive the uncoiling of the strand. Both eukaryotic and viral topoisomerase 1B enzymes contain a highly conserved active site consisting of five common amino acid residues. These residues are Tyr, Arg, Arg, Lys, His/Asp. | In the above figure, [A] shows the C-terminal and N-terminal domains of eukaryotic topoisomerase 1B. [B] shows the C-terminal and N-terminal domains of viral topoisomerase 1B. Both domains are highly conserved between the different species, however, the N-terminal domain of viral topoisomerase 1B is slightly smaller and more simple than the larger eukaryotic N-terminal domain. The type 1B topoisomerase can relieve negative or positive supercoiling without the use of ATP. As long as there's torsional strain on the DNA strand from the supercoiling, this is enough potential energy to drive the uncoiling of the strand. Both eukaryotic and viral topoisomerase 1B enzymes contain a highly conserved active site consisting of five common amino acid residues. These residues are Tyr, Arg, Arg, Lys, His/Asp. | ||
| - | [[Image: | + | [[Image:Active_Sites_of_topo.jpg]] |
| + | |||
Researchers have proven that an asparagine residue can replace the histidine residue in the active site of both eukaryotic and viral topoisomerase, and the enzyme will still undergo the same cleavage mechanism. This suggests that the same cleavage and reliagation mechanisms are the same in all topoisomerase 1B’s.Type IB topoisomerase is a key target for research against the spread of smallpox because it is integral for the viruses replication process. The replication of smallpox is complicated since it doesn’t hijack the host’s genetic machinery to reproduce, this makes the disease highly virulent, and hard to specifically target for elimination by antiviral drugs. | Researchers have proven that an asparagine residue can replace the histidine residue in the active site of both eukaryotic and viral topoisomerase, and the enzyme will still undergo the same cleavage mechanism. This suggests that the same cleavage and reliagation mechanisms are the same in all topoisomerase 1B’s.Type IB topoisomerase is a key target for research against the spread of smallpox because it is integral for the viruses replication process. The replication of smallpox is complicated since it doesn’t hijack the host’s genetic machinery to reproduce, this makes the disease highly virulent, and hard to specifically target for elimination by antiviral drugs. | ||
Revision as of 15:45, 16 November 2015
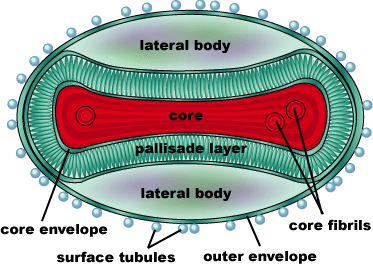
Smallpox (Variola Virus) - Topoisomerase 1B
| |||||||||||
References
Berwald, Juli. "Variola Virus." Encyclopedia of Espionage, Intelligence, and Security. 2004.Encyclopedia.com. 28 Oct. 2015 <http://www.encyclopedia.com>.
"PENN Medicine News: Penn Researchers Determine Structure of Smallpox Virus Protein Bound to DNA." PENN Medicine News: Penn Researchers Determine Structure of Smallpox Virus Protein Bound to DNA. PENN Medicine, 4 Aug. 2006. Web. 28 Oct. 2015. <http://www.uphs.upenn.edu/news/News_Releases/aug06/smlpxenz.htm>.
Perry, Kay, Young Hwang, Frederic D. Bushman, and Gregory D. Van Duyne. "Insights from the Structure of a Smallpox Virus Topoisomerase-DNA Transition State Mimic." Structure (London, England : 1993). U.S. National Library of Medicine, n.d. Web. 28 Oct. 2015. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2822398/>.
“Poxvirus.” Encyclopaedia Britannica. Britannica Academic. Encyclopædia Britannica Inc., 2015. Web. 28 Oct. 2015. <http://academic.eb.com/EBchecked/topic/473442/poxvirus>.
Shubhash, and Parija. "Poxviruses." Textbook of Microbiology and Immunity. Ed. Chandra. India: Elsevior, 2009. 484. Print.
“Smallpox.” Center for Disease Control and Prevention. CDC, n.d. Web. 28 Oct. 2015. <http://www.bt.cdc.gov/agent/smallpox/index.asp>.