Caffeine
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
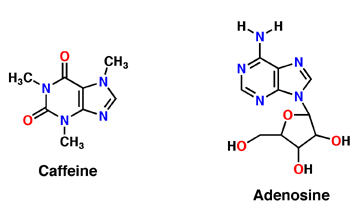
Caffeine is a derivative of adenosine and is also called Trimethylxanthine. It is composed of purines; structurally it is polar, and water soluble. They antagonize or inhibit many of the adenosine receptors, like the A1 receptor mentioned above. Caffeine affects neurons and glial cells in the brain by binding to the same location that adenosine would bind and then induce a cascade of enzymatic downstream effects. | Caffeine is a derivative of adenosine and is also called Trimethylxanthine. It is composed of purines; structurally it is polar, and water soluble. They antagonize or inhibit many of the adenosine receptors, like the A1 receptor mentioned above. Caffeine affects neurons and glial cells in the brain by binding to the same location that adenosine would bind and then induce a cascade of enzymatic downstream effects. | ||
| - | |||
| - | == Relevance == | ||
== Structural highlights == | == Structural highlights == | ||
| Line 25: | Line 23: | ||
Caffeine is a naturally occurring methylxanthine, purine alkaloid, synthesized by eudicot plants such as coffee, cacao, and tea (Denoeud et. al, 2014). In order to synthesize caffeine, xanthosine must undergo 3 methylation steps with the help of three NMT enzymes; xanthosine methyltransferase (XMT), theobromine synthase (MXMT), and caffeine synthase (DXMT) (Denoeud et. al, 2014). The first step of caffeine biosynthesis involves XMT converting S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) which removes a methyl group and adds it to the 7’-Nitrogen. This produces the intermediate 7-methyl-xanthosine which may undergo resonance to become 7-methyl-xanthine (Denoeud et. al, 2014). The second enzyme, MXMT, converts another SAM to SAH, subsequently add a methyl group to the 3’- Nitrogen on 7-methyl-xanthine. This produces theobromine which may undergo another methylation step with the help of the enzyme DXMT. DXMT converts a third SAM to SAH, adding a methyl group to the 1’-Nitrogen, yielding a caffeine molecule (Denoeud et. al, 2014). | Caffeine is a naturally occurring methylxanthine, purine alkaloid, synthesized by eudicot plants such as coffee, cacao, and tea (Denoeud et. al, 2014). In order to synthesize caffeine, xanthosine must undergo 3 methylation steps with the help of three NMT enzymes; xanthosine methyltransferase (XMT), theobromine synthase (MXMT), and caffeine synthase (DXMT) (Denoeud et. al, 2014). The first step of caffeine biosynthesis involves XMT converting S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) which removes a methyl group and adds it to the 7’-Nitrogen. This produces the intermediate 7-methyl-xanthosine which may undergo resonance to become 7-methyl-xanthine (Denoeud et. al, 2014). The second enzyme, MXMT, converts another SAM to SAH, subsequently add a methyl group to the 3’- Nitrogen on 7-methyl-xanthine. This produces theobromine which may undergo another methylation step with the help of the enzyme DXMT. DXMT converts a third SAM to SAH, adding a methyl group to the 1’-Nitrogen, yielding a caffeine molecule (Denoeud et. al, 2014). | ||
| - | == | + | == Trimethylxanthine == |
A2A is a transmembrane G protein in humans. Trimethylxanthine has a highly water soluble and thus when present in the system, interacts with the A2A receptor. In order for Trimethylxantine to bind to the receptor, the third and seventh transmembrane helical domains need to recognize the ligand. Trimethylxanthine can then bind. Trimethylxanthine can bind with very little discomfort, due to its similar structure, as well as its purine alkaloid structure, to adenosine. This binding will change the shape and not initiate the cascade of downstream effects that adenosine does, like opening of ion channels and slowing of activity. Concentrate of free adenosine increases extracellularly, when trimethylxanthine is bound. The cAMP increases when adenosine is bound and (). ERK1 and ERK2 are kinases, composed of serine and threonine, of the GMGC group that regulation of cell growth and differentiation, and if adenosine was bound, this cascade of events would occur, but when Trimethylxanthine is bound, this regulation does not occur. | A2A is a transmembrane G protein in humans. Trimethylxanthine has a highly water soluble and thus when present in the system, interacts with the A2A receptor. In order for Trimethylxantine to bind to the receptor, the third and seventh transmembrane helical domains need to recognize the ligand. Trimethylxanthine can then bind. Trimethylxanthine can bind with very little discomfort, due to its similar structure, as well as its purine alkaloid structure, to adenosine. This binding will change the shape and not initiate the cascade of downstream effects that adenosine does, like opening of ion channels and slowing of activity. Concentrate of free adenosine increases extracellularly, when trimethylxanthine is bound. The cAMP increases when adenosine is bound and (). ERK1 and ERK2 are kinases, composed of serine and threonine, of the GMGC group that regulation of cell growth and differentiation, and if adenosine was bound, this cascade of events would occur, but when Trimethylxanthine is bound, this regulation does not occur. | ||
Revision as of 02:49, 17 November 2015
Caffeine
| |||||||||||
References
Proteopedia Page Contributors and Editors (what is this?)
Mackenzie Kincer, Sidiqullah Halim, Alexander Berchansky, Michal Harel