Caffeine
From Proteopedia
| Line 16: | Line 16: | ||
| + | |||
| + | == Mechanism of Caffeine Synthesis == | ||
| + | |||
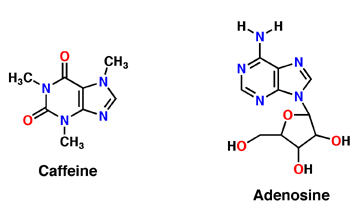
| + | Caffeine is a naturally occurring methylxanthine, purine alkaloid, synthesized by eudicot plants such as coffee, cacao, and tea (Denoeud et. al, 2014). In order to synthesize caffeine, xanthosine must undergo 3 methylation steps with the help of three NMT enzymes; xanthosine methyltransferase (XMT), theobromine synthase (MXMT), and caffeine synthase (DXMT) (Denoeud et. al, 2014). The first step of caffeine biosynthesis involves XMT converting S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) which removes a methyl group and adds it to the 7’-Nitrogen. This produces the intermediate 7-methyl-xanthosine which may undergo resonance to become 7-methyl-xanthine (Denoeud et. al, 2014). The second enzyme, MXMT, converts another SAM to SAH, subsequently add a methyl group to the 3’- Nitrogen on 7-methyl-xanthine. This produces theobromine which may undergo another methylation step with the help of the enzyme DXMT. DXMT converts a third SAM to SAH, adding a methyl group to the 1’-Nitrogen, yielding a caffeine molecule (Denoeud et. al, 2014). | ||
| Line 23: | Line 27: | ||
The adenosine receptor (A2A) is a G-protein, which is a transmembrane protein that consists of secondary structures, such as seven alpha helical domains. Inside the third and seventh transmembrane helical domains, there are hydrophobic side chains that are required for ligand recognition. The target ligand, adenosine, is a large, polar molecule that binds to the extracellular binding domain of the A2A receptor by several nonpolar interactions. To be specific, these nonpolar interactions include hydrogen bonding (11), aromatic stacking interactions (1), and many van der Waals interactions (Xu et. al, 2011). To avoid the steric interactions between the ribose ring of adenosine and the tryptophan of the enzyme binding pocket, these nonpolar interactions cause conformational changes within the binding cavity, and cause an internal rotation and tilt of the seventh helical domain (Xu et. al, 2011). Other molecules, such as caffeine can also bind to these adenosine receptors. When caffeine binds to this receptor, it inhibits adenosine from binding to the extracellular binding domain of the A2A receptor. | The adenosine receptor (A2A) is a G-protein, which is a transmembrane protein that consists of secondary structures, such as seven alpha helical domains. Inside the third and seventh transmembrane helical domains, there are hydrophobic side chains that are required for ligand recognition. The target ligand, adenosine, is a large, polar molecule that binds to the extracellular binding domain of the A2A receptor by several nonpolar interactions. To be specific, these nonpolar interactions include hydrogen bonding (11), aromatic stacking interactions (1), and many van der Waals interactions (Xu et. al, 2011). To avoid the steric interactions between the ribose ring of adenosine and the tryptophan of the enzyme binding pocket, these nonpolar interactions cause conformational changes within the binding cavity, and cause an internal rotation and tilt of the seventh helical domain (Xu et. al, 2011). Other molecules, such as caffeine can also bind to these adenosine receptors. When caffeine binds to this receptor, it inhibits adenosine from binding to the extracellular binding domain of the A2A receptor. | ||
| - | |||
| - | |||
| - | |||
| - | == Mechanism of Caffeine Synthesis == | ||
| - | |||
| - | Caffeine is a naturally occurring methylxanthine, purine alkaloid, synthesized by eudicot plants such as coffee, cacao, and tea (Denoeud et. al, 2014). In order to synthesize caffeine, xanthosine must undergo 3 methylation steps with the help of three NMT enzymes; xanthosine methyltransferase (XMT), theobromine synthase (MXMT), and caffeine synthase (DXMT) (Denoeud et. al, 2014). The first step of caffeine biosynthesis involves XMT converting S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) which removes a methyl group and adds it to the 7’-Nitrogen. This produces the intermediate 7-methyl-xanthosine which may undergo resonance to become 7-methyl-xanthine (Denoeud et. al, 2014). The second enzyme, MXMT, converts another SAM to SAH, subsequently add a methyl group to the 3’- Nitrogen on 7-methyl-xanthine. This produces theobromine which may undergo another methylation step with the help of the enzyme DXMT. DXMT converts a third SAM to SAH, adding a methyl group to the 1’-Nitrogen, yielding a caffeine molecule (Denoeud et. al, 2014). | ||
== Trimethylxanthine == | == Trimethylxanthine == | ||
Revision as of 03:49, 17 November 2015
Caffeine
| |||||||||||
References
Denoeud, F., L. Carretero-Paulet, A. Dereeper, G. Droc, R. Guyot, M. Pietrella, C. Zheng, A. Alberti, F. Anthony, G. Aprea, J.-M. Aury, P. Bento, M. Bernard, S. Bocs, C. Campa, A. Cenci, M.-C. Combes, D. Crouzillat, C. Da Silva, L. Daddiego, F. De Bellis, S. Dussert, O. Garsmeur, T. Gayraud, V. Guignon, K. Jahn, V. Jamilloux, T. Joet, K. Labadie, T. Lan, J. Leclercq, M. Lepelley, T. Leroy, L.-T. Li, P. Librado, L. Lopez, A. Munoz, B. Noel, A. Pallavicini, G. Perrotta, V. Poncet, D. Pot, Priyono, M. Rigoreau, M. Rouard, J. Rozas, C. Tranchant-Dubreuil, R. Vanburen, Q. Zhang, A. C. Andrade, X. Argout, B. Bertrand, A. De Kochko, G. Graziosi, R. J. Henry, Jayarama, R. Ming, C. Nagai, S. Rounsley, D. Sankoff, G. Giuliano, V. A. Albert, P. Wincker, and P. Lashermes. "The Coffee Genome Provides Insight into the Convergent Evolution of Caffeine Biosynthesis." Science 345.6201 (2014): 1181-184.
Proteopedia Page Contributors and Editors (what is this?)
Mackenzie Kincer, Sidiqullah Halim, Alexander Berchansky, Michal Harel